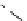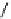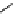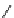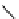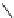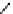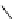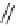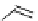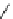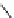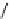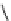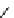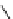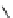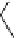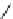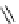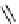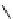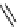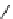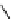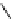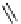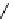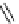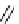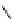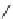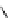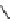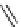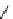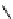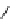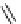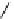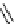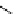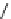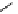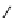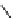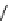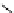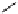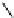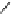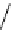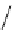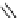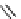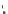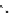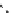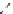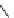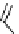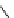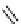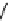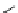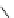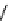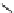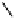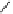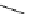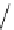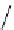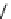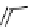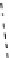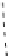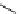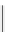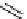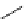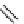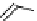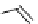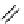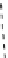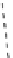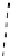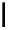Chemistry Reference
In-Depth Information
O
-
O
O
O
-
H
2
N
O
HN
O
O
-
NH
2
O
O
N
O
O
O
O
N
N
O
N
N
O
Gd
N
N
Gd
Gd
O
O
-
O
N
N
N
N
O
-
N
N
O
H
-
O
H
2
N
O
O
O
O
O
O
NH
2
O
NH
O
O
-
O
-
O
[Gd.DOTAM]
3+
[Gd.RRRR-gDOTA]
5-
[Gd.DOTAGly]
-
O
-
O
O
NH
O
O
O
NH
N
O
OH
NH
-
O
O
NH
N
N
OH
N
HN
N
O
N
HO
NH
O
N
N
NH
N
O
Gd
O
HO
N
O
N
N
O
O
HO
O
O
HN
HO
O
O
N
HN
O
N
O
O
-
O
[Gd.RRR-gaDO3A]
3-
tren-1,2-HOPO.H3
tacn-1,2-HOPO.H3
NH
2
O
O
O
O
HO
2
C
CO
2
H
N
CO
2
H
N
N
N
N
N
N
Gd
Gd
O
O
N N
N
N
O
O
CO
2
H
O
O
O
O
AAZTA
Gd.m-XYL
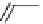
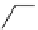
fIgure 8.5
The structures of [gd.dOTAM]
3+
, gd.m-XyL, and complexes/ligands investigated with respect to increasing relaxivity by
increasing τ
M
or q.
co-workers have established that it is possible to inhibit anion and protein binding in such systems: [gd.
RRR
-gadO3A]
3-
(Figure 8.5) exploits a large negative charge to minimise the interactions with ions [58]. In this system, the high relaxivity
observed in water (12.3 mMgd
-1
s
-1
at 20 MHz) is maintained in serum. The bulky chiral pendant arms in this system also
impart additional rigidity to the complex, inhibiting exchange between stereoisomers and giving rise to high kinetic stability.
The authors used the method of Muller and co-workers [59] to screen the kinetic stability of [gd.
RRR
-gadO3A]
3-
by challeng-
ing with endogenous ions and reported that the kinetic stability was ten times greater than that of [gd.dTPA]
2-
. This system
has much appeal because it also offers rapid water exchange (k
ex
= 33 × 10
6
s
-1
), as a consequence of the more open water
binding site and the increased negative charge on the complex (for comparison in gd.dO3A, k
ex
= 6.25 × 10
6
s
-1
) [58].
Other q = 2 systems have also been widely investigated. Raymond and co-workers have used an alternative approach,
focusing on the preference of lanthanide ions for hard donor ligands, and using all oxygen donors [60]. gd.tren-1,2-HOPO