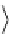Chemistry Reference
In-Depth Information
Values of S
Z
and C
d
are known, and therefore plots of Δω/S
Z
versus C
d
/S
Z
or Δω/C
d
versus S
Z
/C
d
should give straight lines
for iso-structural complexes within a series of lanthanide complexes of the same ligand [33]. Structural variations across a
series of lanthanide complexes are usually ascribed to the gradual decrease in ionic radii owing to the lanthanide contraction.
It is necessary to establish trends in structure across a series because lanthanide ions are often substituted for one another to
allow for useful information to be obtained in the solution state. gd(III) is therefore often substituted for its neighbours in the
f-series, Eu(III) or Tb(III) ions, because they possess useful luminescence characteristics and NMR shifts in a usable range.
For non-symmetric ligands, the polar coordinates ϕ, θ, and r relative to the lanthanide centre can be used to define the
pseudo-contact term:
2
2
2
(
3
cos
θ
−+
1
)
(
sin
φ
cos
2
θ
)
GC
=
(8.5)
r
3
for axially symmetric systems, the second term vanishes [32], so
2
(
3
cos
r
θ
−
1
)
GC
=
(8.6)
3
In both cases,
C
varies with temperature and the nature of the complex (particularly with
B
0
2
, the second order crystal field
parameter, and the nature of the J in the lanthanide state):
CB
kT
β
2
2
=
−2
C
J
0
(8.7)
2
and
2
C
J
=
gJ(J
+
12
)( J (J
−
12
+
3
)JaJ
'
(8.8)
where J is L − S for the early lanthanides and L + S for the later lanthanides in accordance with the Russell-Saunders coupling
scheme. Such parameters may be used to understand and assess the structure of related complexes. In general, the most
effective approach is to ensure that similar structures are adopted by the complexes with (smaller) terbium and (larger) euro-
pium ions. The
1
H NMR spectra of terbium and europium complexes appear very different, because
C
takes the opposite sign
for each ion (this in itself is a simplification, because
C
in europium complexes arises from thermal population of excited J
states in the
7
F
J
manifold - because
C
J
= 0 for J = 0). In the proton NMR spectra of cyclen-derived complexes such as dOTA,
the most shifted proton environment will correspond to one of the axial ring protons (H
ax
) [16]. When we consider the axial
ring protons with reference to known crystal structures [16, 29, 34, 35], a greater magnitude of pseudo-contact shift is always
to be expected for H
ax
(SAP) than for H
ax
(TSAP) because the former subtend a smaller angle (θ) at the molecular axis. In both
cases, H
ax
(SAP) predominates (as can be seen from the marked resonances on the spectra). Thus it may be assumed with
confidence that similar behaviour is exhibited by the gadolinium complexes.
8.3
mInImIsIng the toxIcIty of gadoLInIum contrast agents
Clearly, it is a prerequisite that complexes containing toxic non-endogenous ions that are intended for use
in vivo
must have
high thermodynamic stability if toxicity is to be minimised [5, 6, 18]. All commercial agents fulfil this criterion, and we
have already outlined aspects of their design that can be applied to other systems. It is perhaps less obvious that kinetic
stability is equally important: Unless a system is under kinetic control, even equilibria that involve very strong binding can
be perturbed by kinetic traps such as membrane transport or precipitation. Kinetic stability can be assessed by considering
the rate constants for dissociation or competitive binding. Brücher and co-workers showed that the rate of exchange of
lanthanide ions could be described by an observed rate constant that incorporates pH dependent terms and pH independent
terms [36, 37]:
=+ []
k
k
k
H
(8.9)
exch
ind
dep