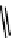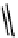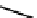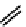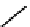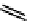Chemistry Reference
In-Depth Information
+
N
N
N
S
67
N
Ga
N
S
N
N
N
18
fIGure 7.11
A monocationic octahedral gallium-67 complex of the APTSm
2
ligand studied
in vivo
.
complexes (which showed IC
50
values ranging from 0.81 to 9.57 mm range against RT2 cells and from 3.6 to 11.30 mm against
T98 cells). These compounds were found to be 20-fold more potent than
cis
-platin and to induce cell death by apoptosis [72].
Due to the interesting anti-proliferative properties of gallium-thiosemicarbazone complexes of this family, [
67
Ga]-labelled
2-acetylpyridine 4,4-di-me thiosemicarbazone, APTSm
2
(Figure 7.11) was also investigated for SPECT imaging applica-
tions. Radiolabelling was performed by treating fresh [
67
Ga]GaCl
3
with the free ligand for 60 minutes at 90° to yield
[
67
Ga(APTSm
2
)
2
]
+
with an almost quantitative radiochemical yield and a specific activity of approximately. 370-740 mBq/
mmol (10-20 Ci/mmol). The high stability in human serum (at 37 °C) led to SPECT and biodistribution studies of the [
67
Ga]
2-acetylpyridine 4,4-dimethyl thiosemicarbazonate complex in normal mice. These were higher than the uptake of free Ga
3+
cation, and it was suggested that this compound represents a promising agent for the detection of malignancies [65, 73].
7.2.7 n
4
donor porphyrin ligands
Porphyrins form extremely stable metal complexes that are highly resistant to demetallation
in vivo
and have therefore been
of interest as radiolabelling hosts. The majority of the research efforts involving medical applications of porphyrins have
been directed toward specific targeting for Photodynamic Therapy (PDT) through conjugation with biomolecules. However,
67
Ga and
111
In radiolabelled porphyrins have also been studied and conjugated to biologically active molecules such as anti-
bodies, antibody fragments, peptides, and proteins to achieve specific targeting.
The reaction between InCl
3
or GaCl
3
with tetraphenylporphyrins gives five-coordinate complexes of the type
[mCl(porphyrin)] (m = Ga, In) where the metal ion sits above the plane of the ligand [74]. The complex [
111
InCl(TTP)]
(TTP = tetraphenylporphyrin) has been prepared and was shown to be completely stable in the presence of serum proteins
[75]. The metallation process occurs under much milder conditions (30 minutes at 25 °C) than those for Tc and Re discussed
previously. One advantage of the porphyrin complexes of Ga and In is that they are strongly fluorescent, which permits
cellular distributions to be determined using confocal fluorescence spectroscopy.
The
67
Ga and
111
In radiolabelling of tetraphenylporphyrin derivatives has also been achieved with further functionalities such
as sulphonate, pyridinium, and anilinium located at the periphery of the porphyrin phenyl groups to modulate solubility and
lipophilicity. Following
in vivo
imaging with these functionalised labelled porphyrins, the accumulation of
111
In and
67
Ga in the
liver and kidneys was observed in all cases, but the blood clearance was notably slower with the sulphonate derivatives.
The InTTAP complex (Figure 7.12) has been shown to give a highly favourable lymph node to muscle uptake ratio of 85:1
[76]. Similarly, InTmPyP (Figure 7.12) was initially shown to delineate malignant melanoma tumours in hamsters [77, 78],
but subsequent studies indicated strong liver and kidney uptake in human carcinoma models [79].
The radiolabelling of TPP with
67
Ga has recently been revisited. labelling was achieved in 30 min. at room temperature
in an acetate buffer, but HPlC revealed the presence of two
67
Ga species. The structures of these were not discussed but may
involve different ligands such as acetate or hydroxide on the Ga. The radiolabelled complexes were very stable in serum, and
biodistribution studies in wild-type rats showed excretion predominantly via the liver [80]. Replacement of one of the pyri-
dyl groups of tetrapyridylporphyrin with a carboxyphenyl group enabled conjugation of the
111
In porphyrin to an anti-CEA
antibody and this was shown to have retained immunoreactivity
in vitro
[81].
In addition, other porphyrin cores based on deuteroporphyrin (Figure 7.12) have been functionalised on the periphery using
conventional chelating groups for isotope labelling. The DTPA ester of Ga4-[1-(2-hydroxy-ethyloxy)ethyl]-2-
vinyldeuteroporphyrin-IX has been radiolabelled with
111
In in greater than 95% radiochemical purity, although is not clear why
the
111
In radiolabel was not simply inserted into the porphyrin. However, the
111
In-DTPA conjugate was subjected to
in vivo
testing in a lewis lung cancer mouse model and showed strong tumour uptake with a tumour/blood ratio of 16.4 +/- 6.6 [82].
Hematoporphyrins (Figure 7.12) have also been labelled with
111
In and examined
in vivo
in mice with induced breast
tumours. malignant tumours showed tumour-to-blood ratios of up to 50 [83]. As for the
99m
Tc complexes (discussed in
Chapter 6) the mechanism of tumour uptake is not known.