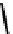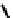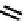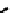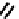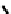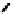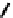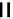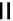Chemistry Reference
In-Depth Information
HO
2
C
O
O
O
O
HN
O
O
Ga
O
Ga
O
O
O
O
O
O
HN
O
O
H
O
CO
2
H
1
2
N
O
MeCONH
N
N
O
O
OH
O
3
OH
3
3
4
fIGure 7.1
ligands that provide an O
6
donor set and Ga complexes.
R
HO
2
C
CO
2
H
NH
HO
2
C
HN
CO
2
H
N
N
SH
HS
HS
SH
R=H, CH
2
C
6
H
4
CO
2
Me
5
6
fIGure 7.2
Some N
2
O
2
S
2
donor ligands.
largely via the liver [11]. Attachment of a carboxymethoxybenzyl group to the ethylene backbone provides a potential
route to bioconjugation. ligand
6
(x = 2), has been encountered in Chapter 6 as the diester where it was used with
99m
Tc as a
myocardial imaging agent. Here, with the free carboxylates, a very stable octahedral Ga complex is formed (log K = 31.5).
The In complex is slightly more stable and also has a distorted octahedral geometry. There are no reports as yet of biological
studies of
67
Ga or
111
In complexes with this ligand system.
7.2.3 n
3
o
3
donor ligands
The neutral [
111
In(oxine)
3
] (oxine = 8-hydroxyquinoline) complex has been used for many years for the labelling of platelets
and white blood cells [12]. Blood samples withdrawn from the patient led to separated white blood cells that were labelled
with [
111
In(oxine)
3
] and injected back into the patient, thereby providing SPECT images of sites of infection. Within cells,
the
111
In is
trans
-chelated, probably to biological iron chelators, leading to irreversible trapping of
111
In [13, 14]. The analo-
gous
67
Ga oxine derivative has not been investigated as a potential radiopharmaceutical. The X-ray crystal structure of
[In(oxine)
3
] has been determined [15]. The indium is, as expected, pseudo octahedral and the 3 N donors are in a
mer
con-
figuration. There was no evidence for other structural isomers.
Gallium forms an extremely stable octahedral complex with NOTA (
7)
(Figure 7.3) [16]. The kinetics of the reaction of
Ga citrate with NOTA have been studied, and the rate determining step is proton reorganisation in a Ga citrate-NOTA
intermediate [17]. The indium NOTA complex is reported to be less robust and has a seven-coordinate structure where the
In retains one chloride and one carboxylate is protonated to give overall neutral charge [16, 18]. Two isomers of NOTA and
related complexes are possible depending on the helicity of the pendant carboxyl groups when coordinated to the Ga. In
the Ga structure discussed above, a single isomer has been formed. However, the potentially hexadentate ligand
8
forms a