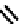Chemistry Reference
In-Depth Information
O
O
OH
O
HO
Tc
Tc
OH
O
O
O
O
P
O
P
O
O
HO
OH
P
OH
O
HO
O
P
O
85
86
fIgure 6.32
HEDP and the structure of Tc methylenediphosphonate complex.
not provide enough incentive for clinicians to change the technetium compounds that are in use or indeed to switch from
PET to SPECT imaging. The way forward might be to exploit the aspects of technetium chemistry that are not available for
PET radionuclides such as
18
F. These might include utilising the ability of Tc in intermediate oxidation states such as (III)
and (IV) to undergo redox reactions at biologically accessible potentials. Hitherto the approach has been to make Tc
complexes that would not oxidise or reduce
in vivo,
but embracing the redox capabilities could create new types of biological
behaviour. The imaging of hypoxia is one obvious example. Technetium-99m also has Auger electron emissions that can be
deployed therapeutically, particularly if targeted to the nucleus of cells where maximal DnA damage can occur. There have
been recent developments of Tc complexes that have both nuclear penetrating peptides and a fluorophore attached to a stable
core that are localised in the nucleus [287, 288]. As the targeting of complexes is refined to the cellular level, the need for
multimodal agents that can be observed by fluorescence microscopy will increase and there is certainly scope here for novel
chemistry. much remains to be discovered about the chemistry of these elements in the context of molecular imaging, and
the coordination chemistry of these elements will surely continue to flourish for the foreseeable future.
references
[1] G. mariani, l. Bruselli and A. Duatti,
Eur.J. Nucl. Med. Mol.Im.
35
, 1560-1565 (2008).
[2] D. Cheng, y. Wang, X. liu, P. Pretorius, m. liang, m. Rusckowski and D. Hnatowich,
Bioconj. Chem.
21
, 1565-1570 (2010).
[3] J. Zeintl, A. Vija, A. yahil, J. Hornegger and T. Kuwert,
J Nucl. Med.
51
, 921-928 (2010).
[4] S. Eshuis, P. Jager, R. maguire, S. Jonkman, R. Dierckx and K. leenders,
Eur.J. Nucl. Med. Mol. Imag.
36
, 454-462 (2009).
[5] m. Santini, A. Fiorelli, G. Vicidomini, P. laperuta, l. Busiello, P. Rambaldi, l. mansi and A. Rotondo,
Respiration
80
, 524-533 (2010).
[6] G. liu and D. Hnatowich,
Anticancer Agents Med. Chem.
7
, 367-377 (2007).
[7] H. Biersack, H. Palmedo, A. Andris, S. Rogenhofer, F. Knapp, S. Guhlke, S. Ezziddin, J. Bucerius and D. von mallek,
J. Nucl. Med.
52
, 1721-1726 (2011).
[8] P. Gould,
Nature
460
, 312-313 (2009).
[9] B. Guerin, S. Tremblay, S. Rodrigue, J. Rousseau, V. Dumulon-Perreault, R. lecomte, J. van lier, A. Zyuzin and E. van lier,
J. Nucl.
Med.
51
, 13n-16n (2010).
[10] S. Qaim,
Nucl. Med. Biol.
27
, 323-328 (2000).
[11] G. Griffiths, D. Goldenburg, F. Roesch and H. Hansen,
Clin. Canc. Res.
5
, 3001-3003 (1999).
[12] H. Bigott, J. Prior, D. Piwinica-Worms and m. Welch,
Mol. Imag.
4
, 30-39 (2005).
[13] E. Watson, m. Stabinand J. Stubbs,
J. Nucl. Med.
35
, 923-924 (1994).
[14] K. Gagnon, S. mcQuarrrie, D. Abrams, A. mcEwan and F. Wuest,
Curr. Radiopharm.
4
, 90-101 (2011).
[15] F. Knapp, A. Beets and S. mirzadeh,
Abstracts of Papers of the American Chemical Society
215
, U934-U934 (1998).
[16] A. J. West,
Ann. Rep. Prog. Chem., Sect. A Inorg. Chem.
105
, 211-220 (2009).
[17] S. R. Banerjee, K. P. maresca, l. Francesconi, J. Valliant, J. W. Babich and J. Zubieta,
Nucl. Med. Biol.
32
, 1-20 (2005).
[18] U. Abram and R. Alberto,
J. Braz. Chem. Soc.
17
, 1486-1500 (2006).
[19] U. Abram,
Comp. Coord. Chem. II
5
, 271-402 (2004).
[20] R. Alberto,
Comp. Coord. Chem. II
5
, 127-270 (2004).
[21] P. Thornton,
Ann. Rep. Prog. Chem., Sect. A Inorg. Chem.
100
, 217-227 (2004).
[22] m. Kohlickova, V. Jedinakova-Krizova and F. melichar,
Chem. Listy
94
, 151-158 (2000).
[23] P. J. Blower and S. Prakash,
Perspect. Bioinorg. Chem.
4
, 91-143 (1999).