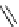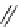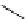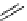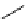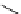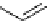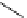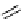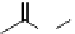Chemistry Reference
In-Depth Information
low-energy β
+
emission provides high-resolution PET images. In light of the challenges caused by the poor solubility of dFO
and its Zr(IV) complexes, the design of novel chelators with improved solubility and chelation properties would be timely.
5.8
124
IodIne nuclIde propertIes
Iodine is unique for this chapter, because it is not incorporated into radiopharmaceuticals through coordination with a BFC
as are the previously discussed metal nuclides. Isotopes of iodine must be covalently attached to molecules in order to be
used in radiopharmaceuticals, much like
18
F (Chapter 3). This provides a different approach to molecular imaging, because
one does not need to attach a bulky biovector such as an antibody or peptide to obtain site-specific delivery (although this
can still be accomplished). For example, the PET imaging nuclide
124
I can be covalently bound to a molecule in place of a
hydrogen atom, as long as the appropriate radioprecursor can be synthesised. Iodine nuclides can also be directly attached
to biovectors such as antibodies (reacted with tyrosine residues) without the attachment of a metal-chelate complex, which
can result in higher immunogenicity of the antibody due to lower steric hindrance, at the cost of modest instability via deio-
dination. Most biovectors function in a classic 'lock and key'-type receptor binding mechanism, and therefore having less
'molecular bulk' (no large BFC metal-complex) helps to retain the biovector's native binding affinity. some of the same
problems encountered in metal-based radiopharmaceutical design are faced with radioiodine-based agents, such as obtaining
high specific activity products, high radiochemical yields and purity, and robust
in vivo
stability.
124
I is typically cyclotron
produced via the nuclear reaction
124
Te(p,n)
124
I [176], and purified by dry distillation [177]. several extensive review articles
have been published on general radioiodination techniques [33, 178], as well as
124
I production and radiosynthesis [27].
5.8.1
clinical trials Based on
124
Iodine
There are a number of FdA-approved radiopharmaceuticals that use
123
I (sPECT, t
1/2
= 13.3 hours),
125
I (sPECT, t
1/2
= 59.4 days),
and
131
I (90% β
-
emission for therapy, t
1/2
= 8 days); however, none are approved that use
124
I (PET, 23% β
+
, t
1/2
= 100.2 hours)
for PET imaging.
122
I is another β
+
emitting isotope that can be used for PET imaging; however, the short half-life of 3.6 min-
utes makes it impractical for most applications. The chemistry of radioiodination is the same for every isotope of iodine, and
so the current FdA-approved offerings could easily be adapted to
124
I derivatives, assuming access to a supply of
124
I. Free
radioiodine can be seen to accumulate in the thyroid and stomach, and sodium iodide (na
123/131
I) is routinely used to image/
treat thyroid cancer, with excess radioiodide being mostly excreted in the urine [179]. Other FdA-approved agents include
123
I-ioflupane (daTscan
TM
, Parkinson's disease diagnosis),
123
I-iobenguane (Adreview
TM
, MIBG, primary or metastatic neu-
roblastoma and pheochromacytoma brain tumours) [180],
125
I-iothalamate (Glofil-125, glomerular filtration evaluation),
125/131
I-hsA (human serum albumin) (Jeanatope/Megatope, blood/plasma imaging), and
131
I-tositumomab (Bexxar
®
, a
labelled antibody that targets Cd20 antigen-expressing non-hodgkin's lymphoma) (Figure 5.15) [51].
Although no FdA-approved
124
I-radiopharmaceuticals are currently available, the clinical trial offerings [53] are
significant (Figure 5.16). The radiolabelled antibody
124
I-cG250 was recently in clinical trials to diagnose renal cancer and
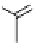
F
O
NH
N
123
I
O
H
NH
2
123
I
123
I-iobenguane
(adreview, MIBG)
123
I-ioupane (DaTscan)
O
HN
I
I
O
O
H
O
-
Na
123/131
I
I
Na
+
125
I-iothalamate (Glol-125)
(When
131
I=HICON)
fIgure 5.15
FdA-approved radioiodine containing radiopharmaceuticals.