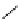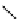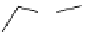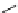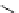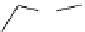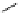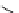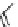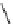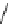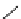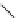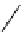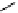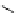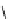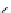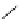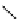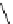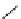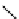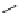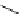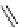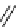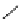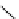Chemistry Reference
In-Depth Information
HO
2
C
HO
2
C
HO
2
C
R
HN
N
N
N
N
O
R
NN
NN
NN
HO
2
C
CO
2
H
HO
2
C
HO
2
C
O
CO
2
H
CO
2
H
NOTA
NODASA-R
NODAGA-R
HO
2
C
NCS
HO
2
C
HO
2
C
NCS
H
N
N
NH HN
NN
NN
HO
2
C
CO
2
H
HO
2
C
CO
2
H
TACN
p
-SCN-Bn-NOTA
nNOTA
fIgure 5.9
The macrocyclic chelating agent TACn and the TACn derivative nOTA, which is currently the 'gold standard' for gallium
complexation, along with several nOTA-based BFC derivatives (
r
= biovector).
ideal for Ga(III) and only binds in a hexadentate fashion (n
4
O
2
) instead of utilising the full denticity of dOTA (octadentate
n
4
O
4
) [103]. Radiometallation of dOTA with
68
Ga requires heating for 30-60 minutes, which is not optimal for a short
half-life isotope [103]. nOTA coordinates Ga(III) with optimal hexadentate (n
3
O
3
) denticity, forming a cavity size that is a
near perfect fit as observed by solid-state X-ray crystallography [101]. The acid inertness of the Ga(III)-nOTA complex is
superb, surviving more than 6 months in 5 M hnO
3
[101]. Many BFC derivatives of nOTA have been synthesised, both
through
N
- and
C
-functionalisation [104]. The BFC derivatives nOdAsA [105] and nOdAGA [106] retain the full hexa-
dentate coordination sphere of nOTA and show the same radiometallation and stability properties as nOTA. despite these
details, a majority of Ga(III)-based radiopharmaceuticals that are translated to the clinic are based on dOTA. An example of
this is the prostate cancer targeting
68
Ga-dOTA-bombesin bioconjugate, which was also studied with
177
lu for therapeutic
applications [95]. The widespread use of dOTA-based BFCs for isotopes such as
64
Cu/
86/90
Y/
177
lu/
89
Zr/
111
In means that a
plethora of radiochemical and clinical data and protocols are available in the literature, making isotopic translation to
68
Ga
more streamlined than with the less investigated, although superior, chelator nOTA. In the future, this trend will hopefully
shift toward chelators that provide optimal stability and coordination properties.
largely due to the development of the attractive
68
Ga generator system, there has recently been a surge in development
of novel BFC agents for
68
Ga. The acyclic chelator hBEd shows exceptional thermodynamic stability with Ga(III), with
a stability constant of log
K
Ml
= 38.5 (Figure 5.10) [107]. hBEd is an attractive acyclic chelator for Ga(III) because of its
fast room temperature radiometallation kinetics, and the BFC derivative hBEd-CC has been conjugated to various anti-
bodies and peptides [108, 109]. The TACn-based chelator TACn-TM is a thiol-containing macrocycle and, although it is
stable with Ga(III), it has not garnered much attention for BFC derivatisation [110, 111]. several promising new
68
Ga
chelators PrP9 (TRAP) [112], PCTA [113], CP256 [114], and h
2
dedpa [115, 116] have been recently published; however,
these examples have only been reported in the last two years and will require further investigation before mainstream
adoption can occur (Figure 5.10). The acyclic chelators h
2
dedpa and CP256 and the dOTA-based chelator PCTA have
demonstrated fast room temperature radiometallation kinetics with
68
Ga; however, PrP9 (TRAP) requires elevated tem-
peratures to achieve quantitative radiolabelling. A unique and novel nOdAGA-like
68
Ga-nOTA-bisphosphonate
(nOTA-BP) complex was recently synthesised as a bone-seeking agent that demonstrated high stability and high affinity
for hydroxyapatite [117].
5.5.3
stability of
68
gallium-Based radiopharmaceuticals
due to the very similar size (62 pm and 65 pm, respectively [15]) and identical charge of the gallium(III) and iron(III) ions,
the iron transport protein transferrin has a very high binding affinity for Ga(III) and a strong tendency to extract it from
weaker chelates [38]. Transferrin is a large protein (~79 kd) with two hydrophilic binding sites, one each at the
C
-terminus
and
N
-terminus [118, 119]. Each of the two binding sites coordinates metal ions with two tyrosine, one histidine, and one
aspartic acid amino acid residues (carbonate binds synergistically and is a required cofactor in iron binding) [118, 119].
The problem of trans-chelation is most pressing for gallium-based radiopharmaceuticals due to the high binding affinity of