Environmental Engineering Reference
In-Depth Information
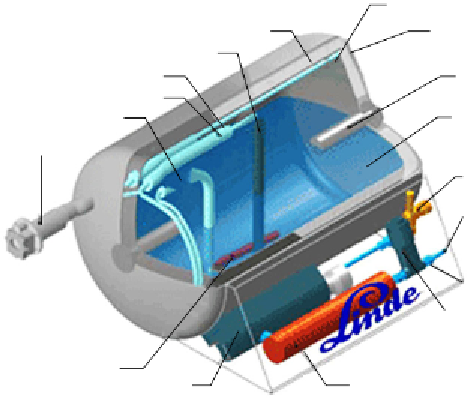
LH2-Tank System
inner vessel
outer vessel
super-insulation
level probe
filling line
suspension
gas extraciton
liquid Hydrogen
(-253°C)
liquid extraction
filling port
safety value
gaseous Hydrogen
(+20°C up to +80°C)
shut-off value
electrical heater
reversing value
(gaseous / liquid)
cooling water
heat exchanger
www.Linde.com
FIGURE 5.10
Schematic illustration of a representative cryogenic vessel. Source: “Hydrogen
storage: state-of-the-art and future perspective,” http://publications.jrc.ec.europa.eu/repository/bitstream/
111111111/6013/1/EUR%2020995%20EN.pdf. (See color insert.)
energy, also have different thermal dynamic properties. The parahydrogen
has lower melting and boiling points than those of the orthohydrogen. When
hydrogen is cooled down, more orthohydrogen is converted to parahydrogen.
The ratio of orthohydrogen can be reduced from 75% at room temperature
to 25% at 77 K, and can be further reduced to 0.2% when the hydrogen is
cooled down to the boiling point (20.08 K). The conversion of orthohydrogen
to parahydrogen is a heat-release process. As long as there are orthohydro-
gens in the cryostats, conversion of orthohydrogen to parahydrogen is inevi-
table, which will cause heating of the liquid hydrogen. Clearly, although
liquid hydrogen has significantly higher energy capacities compared with
compressed hydrogen, it has some disadvantages, mainly the large amount
of energy required to liquefy hydrogen, the strict requirements of cryogenic
vessels with complicated thermal and pressure management, and hydrogen
losses through evaporation from the containers.
5.4 SUMMARY
The compressed hydrogen tank and liquid hydrogen are the two most popular
hydrogen storage methods for current industrial use. To use them as hydro-
gen vehicle, they will face similar challenges, such as vessel design and
Search WWH ::

Custom Search