Environmental Engineering Reference
In-Depth Information
(a)
(b)
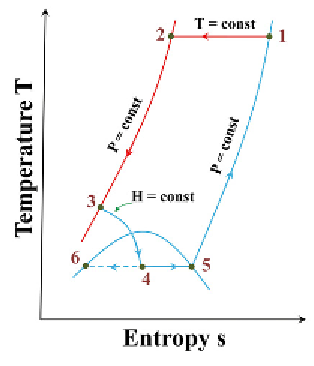
FIGURE 5.8
Schematic and temperature-entropy diagram of a simple Linde cycle.
Source
: Reproduced
with permission from Barron [11]. (See color insert.)
Between 60% and 80% of the gas is then deviated from the mainstream,
expanded through an expander. Such an expansion process is isentropic and
a much lower temperature is attained than from an isenthalpic expansion.
The portion of gas is reunited with the return stream below the second heat
exchanger. The stream to be liquefied continues through the second heat
exchanger, the third heat exchanger, and is finally expanded through a JT
valve to the liquid tank. The cold vapor from the liquid tank is returned
through the heat exchangers to cool the incoming gas. The Claude cycle may
be used without modification to liquefy hydrogen since the system does not
primarily depend on the expansion valve to produce low temperatures. In
addition, by using liquid nitrogen precooling with the Claude system, a figure
of merit 50-70% higher than that of the precooled Linde system may be
obtained. Other methods, such as Haylandt cycle and dual-pressure Claude
cycle, can be used to liquefy hydrogen also [11].
Liquid hydrogen needs to be stored in cryogenic vessels (or cryostats).
The cryostats are metallic double-walled vessels with insulation, sandwiched
between the walls. To minimize thermal losses, effects of thermal radiation,

Search WWH ::

Custom Search