Environmental Engineering Reference
In-Depth Information
(a)
(b)
(c)
(d)
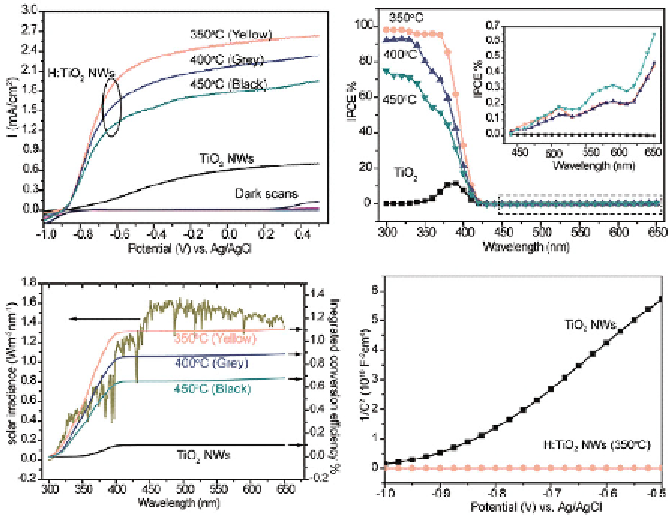
FIGURE 3.7
(a) Linear sweep voltammograms collected on pristine TiO
2
nanowire and hydrogen-
treated TiO
2
(H:TiO
2
) nanowires annealed at temperature of 350, 400, and 450°C. (b) IPCE spectra of
pristine TiO
2
and H:TiO
2
nanowires. The inset is the magnified IPCE spectra that highlighted in the dashed
box. (c) Simulated solar-to-hydrogen efficiencies for the pristine TiO
2
and H : TiO
2
samples as a function
of wavelength, by integrating their IPCE spectra collected at −0.6 V versus Ag/AgCl with a standard
AM 1.5G solar spectrum. (d) Mott-Schottky plots collected at a frequency of 5 kHz in the dark for pristine
TiO
2
and H:TiO
2
nanowire.
Source
: Reproduced with permission from Wang et al. [11]. (See color insert.)
hydrogen treatment at elevated temperatures. Oxygen vacancies serve as
shallow donors that can improve the electrical conductivity of metal oxides
[11, 25]. Notably, hydrogen-treated samples showed substantially increased
photocurrent density, compared with pristine TiO
2
(Fig. 3.7a). A maximum
photocurrent density of around 2.5 mA cm
−2
was obtained for the hydrogen-
treated TiO
2
at 0 V versus Ag/AgCl in 1.0 M NaOH aqueous solution [11].
Incident photon-to-current efficiency (IPCE) analysis suggested that the
enhanced photocurrent was due to improved photoactivity of TiO
2
in the UV
region (Fig. 3.7b). The increased IPCE values were attributed to enhanced
charge collection efficiency as expected for hydrogen-treated TiO
2
that has
improved electrical conductivity. By integrating the IPCE spectra with stan-
dard AM 1.5G spectrum, a simulated maximum solar to hydrogen conversion
efficiency of 1.1% was obtained for hydrogen-treated TiO
2
(Fig. 3.7c).
Search WWH ::

Custom Search