Environmental Engineering Reference
In-Depth Information
(a)
100
Conversion
80
60
40
20
0
1
2
3
4
5
6
7
8
9
10
11
12
13
Time, hrs
(b)
100
80
Conversion
60
40
20
0
1
2
3
4
5
6
7
8
9
10
11
12
13
Time, hrs
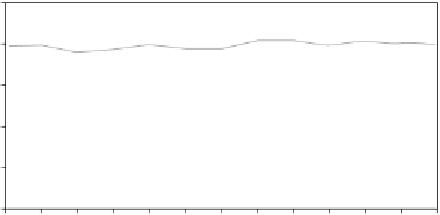
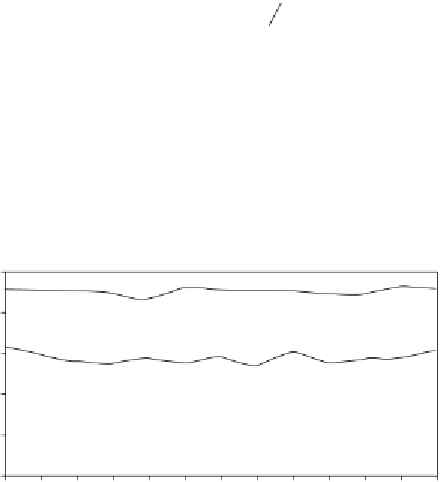
FIGURE 2.6
Hydrogen selectivity and glycerol conversion over (a) Ni/Al
2
O
3
and (b) Rh/CeO
2
/Al
2
O
3
for 13 hours at 900°C, with a feed flow rate of 0.15 mL min
−1
and water to glycerol ratio of 6.
Source
:
Reproduced with permission from Adhikari et al. [14].
usually not necessary to carry out fine purification after ammonia cracking.
Furthermore, co-reactants such as water are not required.
The reaction rate depends on temperature, pressure, and the catalyst used.
The theoretical limit for the lowest working temperature possible is given
by the chemical equilibrium for the dissociation reaction. Figure 2.7
shows that a nearly complete conversion from ammonia to hydrogen and
nitrogen at higher temperatures (near 430°C) and atmospheric pressure is
possible [15].
Catalysts used for ammonia dissociation include materials such as porce-
lain or silica glass, metals such as Fe, W, Mo, and Ni, as well as noble metals
and metal oxides. Active catalytic reactions are usually conducted in the
temperature range from 700 to 1100°C. Commercially available simple cata-
lyst materials such as nickel oxide or iron oxide on aluminum and the influ-
ence of the addition of noble metals such as Pt have been investigated.
The synthesis of ammonia in industry is usually based on the reverse
process of Equation 2.19, that is reaction between hydrogen and nitrogen,


































Search WWH ::

Custom Search