Environmental Engineering Reference
In-Depth Information
50
H
2
CO
CH
4
40
C
2
H
2
/C
2
H
4
C
2
H
6
C
3
H
8
30
20
10
0
0.15
0.20
0.25
0.30
0.35
0.40
0.45
Ethanol flowrate (mL/min)
FIGURE 2.5
Dependence of selectivity of product gases on the flow rate of ethanol.
Source
: Repro-
duced with permission from Wang et al. [10].
dependence of selectivity of product gases on the flow rate of ethanol. The
ethanol conversion efficiency and hydrogen yield increased with the vapor-
ization at room temperature up to the maximum at first, and then decreased
slightly. The maximum hydrogen yield of 31.8% was obtained at an ethanol
conversion of 88.4% under the optimum operation conditions of vaporization
temperature of 120°C, ethanol flux of 0.18 mL min
−1
, water/ethanol ratio of
7.7, and oxygen volume concentration of 13.3%.
2.5 GLYCEROL REFORMING
Glycerol represents another important renewable source for H
2
. With a chem-
ical formula of C
3
H
8
O
3
, it is a saturated and oxygenated hydrocarbon with
one OH group on each of the three carbon atoms and a one-to-one oxygen-
to-carbon ratio. It is edible, biodegradable, nonflammable, nontoxic, and high
boiling. Glycerol can be synthesized from propylene oxide, sorbitol, or
glucose, or obtained as a byproduct in several industrial processes, such
as soap manufacturing, biodiesel production, or lignocellulose-to-ethanol
conversion.
2.5.1 Glycerol Reforming Processes
Glycerol reforming has been extensively studied recently, including steam
or aqueous reforming, catalytic partial oxidation, and autothermal reforming





















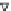
















Search WWH ::

Custom Search