Environmental Engineering Reference
In-Depth Information
SORPTION-ENHANCED SMR REACTION
Gas Phase
CH
4
+ H
2
O
CH
4
+ H
2
O = CO + 3H
2
CO + H
2
O = CO
2
+ H
2
Fuel Cell Grade
Hydrogen
Admixture of CO
2
Chemisorbent + SMR Catalyst
THERMAL SWING REGENERATION
Gas Phase
CO
2
Super Heated
Steam
H
2
O + CO
2
Admixture of CO
2
Chemisorbent + SMR Catalyst
Heat
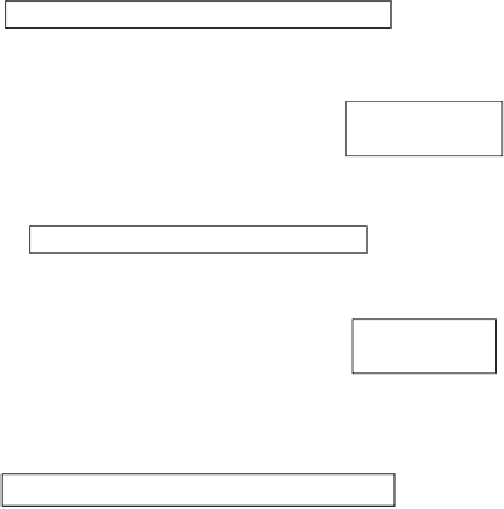
Basic Principles of TSSER - SMR Concept
FIGURE 2.1
Schematic of the thermal swing sorption-enhanced reaction (TSSER)-steam-methane
reforming (SMR) concept.
Source
: Reproduced with permission of Beaver et al. [2].
In a recent study, a new concept based on sorption-enhanced reaction
(SER) for steam-methane reforming (SMR) was demonstrated to directly
produce fuel-cell grade H
2
(∼10 ppm CO) with very high CH
4
to H
2
conver-
sion efficiency (>99%) using an admixture of a commercial SMR catalyst
and a CO
2
chemisorbent, K
2
CO
3
promoted hydrotalcite [2]. The reaction was
carried out at a much lower temperature (520-590°C) than the conventional
reaction temperature of 700-900°C without sacrificing the reactor perfor-
mance. It also eliminates the subsequent H
2
purification step by a conven-
tional PSA process. Figure 2.1 shows a schematic of the thermal swing
sorption-enhanced reaction (TSSER)-steaming methane reforming (SMR)
concept. The overall outcomes are attributed to four related factors: (a) favor-
able thermodynamic equilibrium of the highly endothermic SMR reaction at
the higher reaction temperature, (b) faster kinetics of SMR reaction at higher
temperatures, (c) favorable removal of CO
2
from the reaction zone at lower
temperatures, and (d) higher cyclic working capacity for CO
2
chemisorption
at higher temperature.



























































































Search WWH ::

Custom Search