Environmental Engineering Reference
In-Depth Information
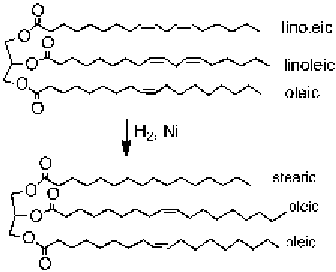
FIGURE 10.7
A schematic diagram illustrates partial hydrogenation of a triacylglycerol. The reactant
is a typical kind of vegetable oil and the product is a typical component of margarine [14].
carbon monoxide, and hydrogen are commonly used as reduction agents.
Among them, hydrogen has been avoided to be used for the production of
metals such as iron, copper, and aluminum due to safety and cost consider-
ations. Nevertheless, hydrogen has been commonly used for the extraction
of two refractory metals, tungsten and molybdenum, because very pure metal
powders can be obtained by hydrogen reduction of their native oxides [15].
For example, production of tungsten metal has almost been carried out
exclusively by hydrogen reduction [16]. The reduction mechanism is depend-
ing on the moisture level in the environment. At low content of moisture, the
reduction proceeds by solid-state diffusion of the oxygen out of the oxide,
and can be represented by the following equation [15]:
WO s
( )
+
3
H g W s
( )
→
( )
+
3
H O g
( ).
(10.6)
3
2
2
Nevertheless, another reaction path will be more favorable under higher
temperature and high content of moisture. First, the oxygen-rich tungsten
compounds will form W
20
O
58
and W
18
O
47
and eventually converted to WO
2
.
The WO
2
will further react with water to form WO
2
(OH)
2
, which can con-
sequently react with hydrogen to produce pure metal tungsten:
WO s H O g WO OH g H g
( )
+
( )
→
(
) ( )
+
( )
(10.7)
2
2
2
2
2
WO (OH) g
( )
+
3
H g W s
( )
→
( )
+
4
H O g
( ).
(10.8)
2
2
2
2
In practice, the grain size of tungsten powder can be controlled empirically
by setting the temperature, oxide quantity, heating time, and H
2
flow rate.
The range of size usually ranges from 0.1 to 60 μm [15].
Search WWH ::

Custom Search