Environmental Engineering Reference
In-Depth Information
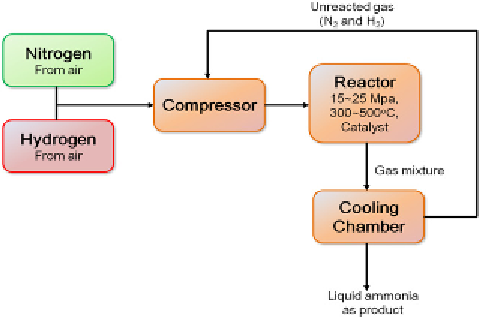
FIGURE 10.6
A flow chart illustration of the main components in a typical Haber process [11].
pollutants such as sulfur and nitrogen from chemical fuels. During this
process, hydrogen gas will be used to hydrogenate sulfur and nitrogen com-
pounds, and finally remove them in the form of H
2
S and NH
3
gases [9].
10.3 HYDROGEN UTILIZATION IN CHEMICAL INDUSTRY
10.3.1 Ammonia Production: The Haber Process
The Haber process, also known as Haber-Bosch process, is the well-
established method for production of ammonia using nitrogen and hydrogen
as precursor gases. It was first reported by two German scientists Fritz Haber
and Carl Bosch in the early twentieth century. This process has been refined
and optimized for industrial-level production of ammonia and other nitrogen
fertilizers. This is one of the most important processes in chemical industry,
and significantly, it consumes almost 50% of the total global hydrogen pro-
duction [9].
Figure 10.6 shows a schematic illustration of the Haber process. The
Haber process takes nitrogen gas from air and combines it with hydrogen
from natural gas to form ammonia:
Catalyst
MPa
→
−
1
N
+
3
H
2
NH
; ∆
H
=−
9
2 22
.
kJ mol
⋅
.
←
(10.1)
2
2
3
15 25
−
,
300 500
~
°
C
Reaction pressure and temperature are typically set to be 15∼25 MPa and
300∼500°C. An optimal conversion efficiency of ∼15% can be achieved in
this single-step reaction. Unreacted gases will be separated from liquid
Search WWH ::

Custom Search