Environmental Engineering Reference
In-Depth Information
FIGURE 10.4
Hagg-Dessau cracking mechanism for an alkane molecule proceeding via a carbonium
ion transition state [7].
Alkyl-cyclohexane
β
-
bond clevage
CH
3
H
2
CH
2
˙
Desorption
Dissociation
H
H
H
2
H
2
˙
˙
˙
H
3
C
S
Mo
S
S
Mo
S
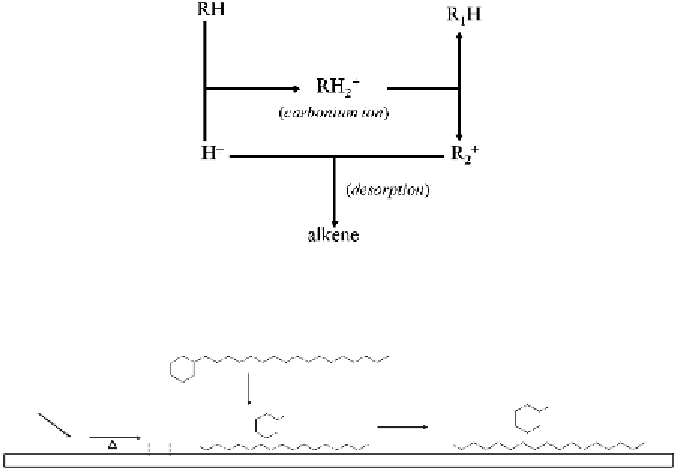
FIGURE 10.5
Proposed mechanism for hydrolysis of alkyl cyclohexanes on MoS
2
catalyst
surface [8].
The fourth example is thermal hydrocracking (or hydropyrolysis). Even
in the absence of a catalyst, thermal hydrocracking can be achieved at ele-
vated temperatures (e.g., 500-600°C) and hydrogen pressure. As shown in
Figure 10.5, the gaseous H
2
is first adsorbed on the surface of MoS
2
and
dissociates into free radical H• when migrating to the Lewis acid sites.
Simultaneously, the alkyl cyclohexanes are produced from
β
-scission of side
chains under increasing thermal stress, probably with the assistance of catal-
ysis of MoS
2
. When the cleaved radical fragments encounters H•, termination
reactions immediately take place to generate smaller molecules involving
alkanes and cyclohexanes, which would be ultimately desorbed and expelled
from the surface of catalyst [8].
10.2.2 Hydroprocessing
Petroleum products and natural gas typically contain a large amount of sulfur
and nitrogen. The presence of sulfur and nitrogen impurities not only lowers
the quality of the fuels but also causes severe air pollution by forming NO
x
and SO
x
gases during their combustion. These oxide gases are the major
sources of acid rain. Hydroprocessing is the method designed to remove
Search WWH ::

Custom Search