Environmental Engineering Reference
In-Depth Information
0.5
: USY(7)
: MFI(25)
: MOR(10)
: MOR(64)
: MOR(100)
0.4
0.3
0.2
0.1
0
0.1
1
10
100
Pressure (bar)
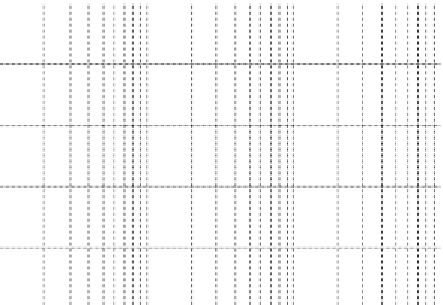
FIGURE 7.6
Hydrogen adsorption isotherms of various zeolites at 30°C.
Source
: Reproduced with
permission from Chung [42].
In another study, the roles of the framework structure, surface area, and
pore volume of microporous zeolites on hydrogen adsorption have been
investigated using a high pressure dose of hydrogen at 30°C [42]. Figure 7.6
shows representative hydrogen adsorption isotherms on different micropo-
rous zeolites, which reached equilibrium after being dosed with 50 bar of
hydrogen. The largest hydrogen adsorption was approximately 0.4 wt% on
USY(7) zeolite. Although this storage capacity is insufficient to the target of
DOE, it can be considered as a storage material of hydrogen with its modi-
fication by ion exchange and enlargement of pore volume, because the zeo-
lites have a large pore volume and suitable channel diameter close to kinetic
diameter of the hydrogen molecule (2.89 Å). The amount of hydrogen
adsorption on mordenite (MOR) zeolites increased with increasing Si/Al
molar ratio, which was achieved by dealumination. The amount of hydrogen
adsorption increased linearly with increasing pore volume of the zeolites.
The hydrogen adsorption behavior was found to be dependent mainly on the
pore volume of the zeolites.
7.2 PHYSICAL STORAGE USING METAL-ORGANIC FRAMEWORKS
Metal-organic frameworks (MOFs) are a unique class of synthetic porous
materials that have been demonstrated to store hydrogen. Due to the special
characters of MOFs, we will discuss them in a separate section here.








































































Search WWH ::

Custom Search