Environmental Engineering Reference
In-Depth Information
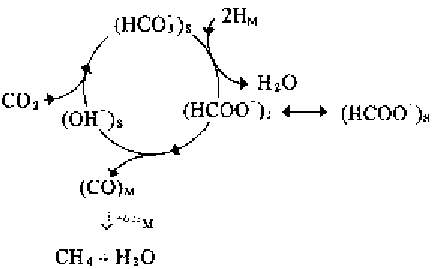
FIGURE 6.16
Schematic illustration of the CO
2
methanation process via hydrogenation. S stands for
the support, M for the metal, and I for the metal-support interface.
Source
: Adapted from Marwood
et al. [50].
For direct methanol generation from CO
2
hydrogenation, the reaction is
given as:
CO
+
3
H
→
CH OH H O H
+
∆
=−
49 5
.
kJ mol
⋅
−
1
.
(6.19)
2
2
3
2
298
K
Based on thermodynamics, a decrease in temperature and an increase in
pressure would favor methanol formation. By-products for methanol synthe-
sis include CO, hydrocarbons, and higher alcohols. Thus, a highly selective
catalyst is needed to avoid the formation of undesired by-products for metha-
nol synthesis. Among the many metal-based catalysts, Cu remains the
primary active catalyst component, together with various modifiers, such as
Zn, Zr, Al, Ce, Si, Ti, B, and Cr. The catalysts are usually dispersed on oxide
supports, such as ZnO and ZrO
2
, which can play an important role in the
reaction by affecting the formation and stability of the catalysts, as well as
interaction between catalysts and promoters. Methanol selectivity is strongly
dependent on the specific catalysts and supports used, and in some cases,
near 100% selectivity is reached [55]. Despite extensive studies, the reaction
mechanism of methanol synthesis is still not well understood. One model
suggests that the reaction is occurring at the interfaces of Cu and oxides with
CO
2
adsorbed on the oxides and H
2
dissociating on Cu [56].
6.5 SUMMARY
Metal hydrides represent one of the most promising materials for hydrogen
storage. They usually have very high volumetric density but low gravimetric