Environmental Engineering Reference
In-Depth Information
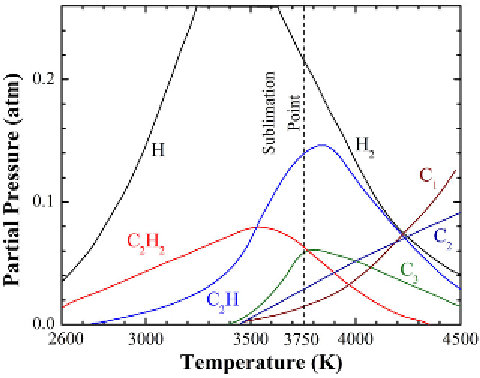
FIGURE 6.13
Equilibrium diagram of the C + H
2
system at 1 atm pressure.
Source
: Adapted with
permission from Baddour and Iwasyk [39]. (See color insert.)
amount of C
2
H
6
and C
3
H
8
. The content of formed hydrocarbon increased
with temperature.
A more recent study examined the interaction between carbon and hydro-
gen atoms on a Ru(0001) surface using scanning tunneling microscopy
(STM), density functional theory (DFT), and STM image calculations [41].
Formation of CH species by reaction between the adsorbed H and C was
observed to occur readily at 100 K. When the coverage of H was increased,
new complexes of the form of CH +
n
H (
n
= 1, 2, 3) were observed. These
complexes were suggested as possible precursors for further hydrogenation
reactions. DFT analysis indicated a considerable energy barrier for the
CH + H → CH
2
reaction.
6.4.2 Reaction between Solid Carbon and Hydrogen
The reaction between carbon atom and hydrogen atom or molecule serves
as a first step toward understanding reaction between solid carbon materials
such as graphite, graphene, carbon nanotubes, and fullerenes. The interaction
between hydrogen and solid carbon materials can vary from physical (weak)
to chemical (strong) in nature. This topic will be addressed partly in Chapter
7, which focuses on various physical storage materials including carbon-
based materials.
In this section, we will address briefly chemical reactions between solid
carbon materials and hydrogen. While the detailed structures of the carbon
Search WWH ::

Custom Search