Environmental Engineering Reference
In-Depth Information
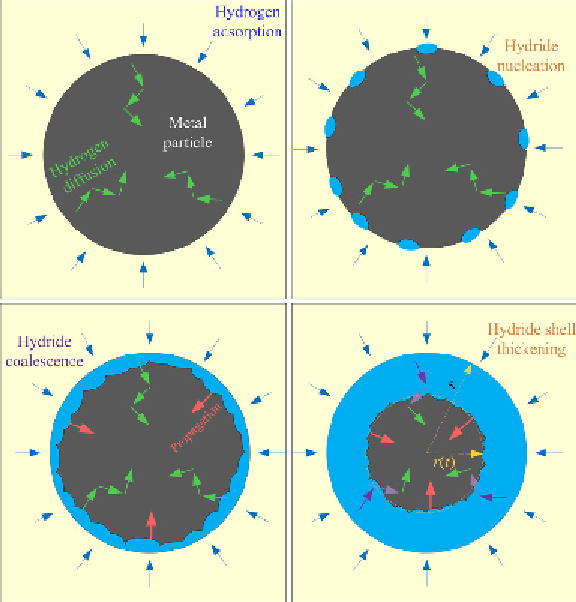
FIGURE 6.5
Illustration of the four stages of hydrogenation process for a metal powder. (See color
insert.)
[5]. Initially, the hydrogen will adsorb on the metal surface and diffuse into
the metal. At appropriate temperature, hydrogen starts to react with the
metal to form metal hydride. The reaction starts at the surface of the metal,
and propagates into the metal core. At the beginning, random hydride
patches will be formed on the metal surface, similar to a nucleation process.
With the progress of the reaction, the hydride patches will coalescence and
form a hydride shell around a metal particle, and subsequent reactions will
involve propagation of this hydride shell. The formation of the hydride shell
also has another negative effect, that is, to impede the hydrogen diffusion
from the atmosphere into the inner core of the particle (the hydrogen diffu-
sion rate in metal is in general much faster than that in metal hydride).
Therefore, multiple rate limit processes could determine the hydrogenation
kinetics.
Search WWH ::

Custom Search