Environmental Engineering Reference
In-Depth Information
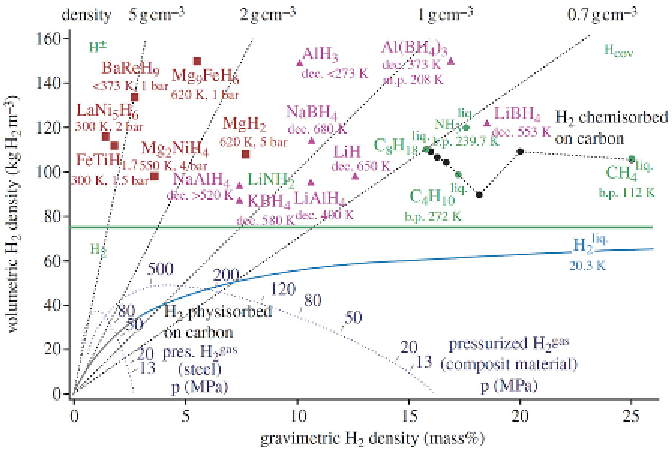
FIGURE 6.1
Volumetric and gravimetric hydrogen density of some selected hydrides.
Source
: Repro-
duced with permission from Züttel et al. [1].
required to release the hydrogen, which presents a major challenge for metal
hydrides to meet the target for onboard hydrogen fuel systems, that is,
<100°C for hydrogen release and <700 bar for hydrogen recharge
(20-60 kJ·mol
−1
H
2
). In addition, all the metal hydrides that can operate at
ambient temperature and pressure consist of transition metals and have a
relatively low gravimetric density (usually <10 wt%). Therefore, exploring
lightweight metal hydride and tuning their thermodynamic and kinetic prop-
erties are of strong interest.
6.2 HYDROGEN STORAGE CHARACTERISTICS OF
METAL HYDRIDES
Since the hydrogen storage mechanisms of metal hydrides and complex
hydrides involve primarily chemical reaction processes, their storage
characteristics are more complicated than that of physical storage of pure
hydrogen discussed in Chapter 5. To determine whether the material has
good hydrogen storage performance, the following properties need to be
examined.
Search WWH ::

Custom Search