Biomedical Engineering Reference
In-Depth Information
Others searched for osteogenic stem cells in
tissues such as skin, muscle, and fat [
size bone matrix in vitro [
37
,
125
] and in vivo
5
,
6
,
33
,
50
,
[
A). Even though adipose-
derived stem cells (ADSCs) constituted an abun-
dant alternative to BM-MSCs, it was necessary
to do some ex vivo expansion prior to utilizing
them in vivo [
23
,
38
,
40
,
92
] (Fig.
1
.
2
51
]. Of these, adipose tissue
appeared to be the most promising, both eco-
nomically and practically. Adipose tissue is
abundant and relatively easy to harvest, and the
number of stem cells that can be harvested from
it is two to three log units higher per number of
isolated cells than is the case for BM-MSCs.
When the number of these cells was increased,
they differentiated into osteoblasts that synthe-
,
104
,
112
,
125
]. Recent data from
our laboratories suggest that freshly isolated
ADSCs may be loaded directly onto osteo-
supportive matrices and can form bone in vivo
(Fig.
23
,
38
,
40
,
92
B). However, this treatment strategy
still needs rigorous testing.
1
.
2
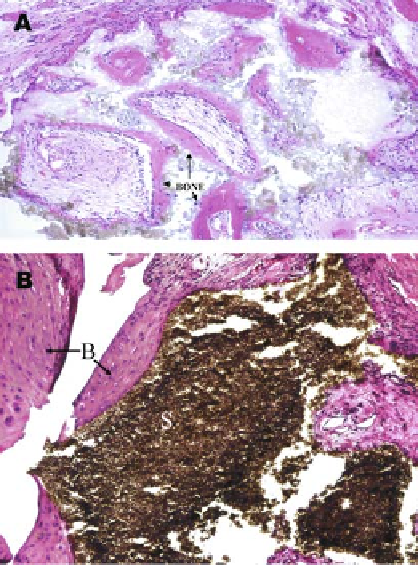
Figure 1.2.
Bone formation by adipose-derived stem cells
(ADSCs). Human ADSCs can generate new bone when implanted
subcutaneously into immunodeficient mice (A). The cells were
loaded onto hydroxyapatite scaffolds prior to implantation and
were retrieved after 6 weeks. The scaffolds were processed and
stained with hematoxylin and eosin. Noncultured ADSCs syn-
thesized new bone (B with arrows) adjacent to the implanted
scaffold (S) in a rat critical size defect (B). In the only case reported
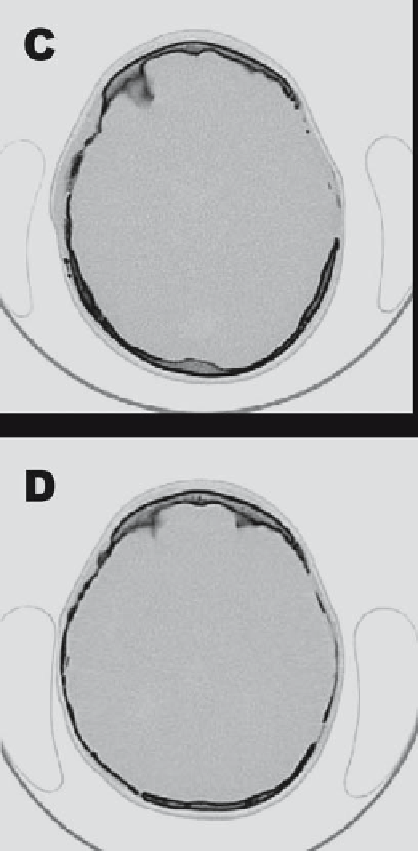
to date, primary ADSCs mixed with autograft were used to treat
severe cranial defects in a nine-year-old female patient. Axial CT
scans of her skull before surgery (C) and 3 months after surgery (D)
reveal that significant mineralization has occurred in the defect
site. Reproduced with permission from Lendeckel et al. [67].