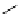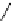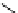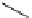Chemistry Reference
In-Depth Information
helices adopted an excess one-handed helical conformation in solution as well as in the solid
state. By extending this molecular strategy, double helices bearing platinum(II) linkers,
which undergo the double helix-to-double helix transformations through the chemical
reactions of the platinum(II) complex moieties, were synthesized (Scheme 3.48).
Furthermore, optically active double helixes are synthesized through a twist-sense bias
induced by a chiral phosphine ligand on one of the complementary metallostrands followed
by a ligand exchange reaction with an achiral amidine ligand, which replaces the chiral
ligand, to bridge the two strands [93]. The catalyst can efficiently induce asymmetry in the
cyclopropanation reaction of styrene and ethyl diazoacetate.
3.4.3 Reagent Source
A seven-membered amidinium compound can be synthesized from 2,2
0
-diaminobiphenyl
as a seven-membered heterocyclic carbine precursor [94] (Figure 3.10).
Cyclic imidazolinium salts are prepared from acylic amidines through intramolecular
hydroamidiniumation of alkene. Thus, heating alkenyl amidinium salts, obtained by treating
alkenylamidines with a stoichiometric amount of hydrogen chloride, induces ring closure
regioselectively via exo addition of the nitrogen-hydrogen bond to the pendent carbon-
carbon double bond, to give cyclic imidazolinium salts [95] (Scheme 3.49). This synthetic
method is easily accessible to 4,4-disubstituted imidazolidinium salts, potential precursors
for novel N-heterocyclic carbenes and applicable to the synthesis of cyclic iminium salts.
R
N
R = neopentyl, 2-adamantyl
H
+
N
BF
4
-
R
Figure 3.10
Structures of seven-membered amidinum compounds
R
R
H
NN
Ar
1. BuLi
HCl
H
Ar
NN
Ar
+
NN
Ar
R
2.
Ar
Ar
89%
Br
ex. Ar = 2,6-di
i
PrC
6
H
3
R
Me
110 ˚C
Cl
-
NN
Ar
+
Ar
24 h
80%
Scheme 3.49
Preparation of cyclic imidazolidinium salts from acyclic amidines