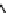Chemistry Reference
In-Depth Information
DBU (
1
)
(1 equiv)
R
1
R
1
NO
2
O
R
2
R
2
MeCN
R
1
, R
2
= alkyl
Scheme 3.42
DBU (1) mediated Nef reaction of nitroalkanes
R
1
O
-
H
N
DBU (
1
)
N
R
1
R
1
O
OH
R
2
O
-
N
R
2
N
N
R
2
O
-
O
R
1
R
1
- HNO
R
2
NO
O
R
2
HO
Scheme 3.43
Proposed reaction mechanism for DBU (1) mediated Nef reaction
3.3.17 Nucleophilic Epoxidation
DBU (1) effectively catalyses the epoxidation of a range of enones derived from tetralone or
related cyclic ketones using poly-
L
-leucine and urea-hydrogen peroxide (H
2
O
2
) in isopro-
pyl acetate [84]. Epoxides were obtained in 63-85%yield and 59-96% ee [84b] (Table 3.7).
Table 3.7
DBU (1) catalysed asymmetric epoxidation
O
urea-H
2
O
2
, (0.12 mmol)
DBU (
1
) (0.20 mmol)
O
O
R
R
poly-L-leucine (200 mg)
isopropyl acetate (1.6 cm
3
)
(0.24 mmol)
Run
R
n
Time (min)
Yield (%)
ee (%)
1
Ph
1
90
76
84
2
4-BrC
6
H
4
1
72
81
82
3
Me
1
60
66
92
t
Bu
4
1
192
63
83
5
H
1
7
64
94
6
Ph
0
48
72
88
7
Ph
2
168
74
59