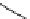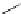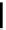Chemistry Reference
In-Depth Information
Table 3.3
DBU (1) catalysed azidation
CO
2
Et
CO
2
Et
azide, DBU (
1
)
OH
N
3
solvent, 50 ˚C, 18 h
97% ee
Azide
a
Run
Solvent
1 (equiv.)
Yield (%)
ee (%)
1
DPPA
toluene
1.2
63
0
2
DPPA
toluene
0.95
61
20
3
(NO
2
)
2
DPPA
toluene
1.2
86
83
4
(NO
2
)
2
DPPA
THF
0.95
60
93
5
(NO
2
)
2
DPPA
DMF
1.2
81
80
6
(NO
2
)
2
DPPA
DMF
0.95
72
95
>
a
DPPA
¼
diphenyl phosphoryl azide; (NO
2
)
2
DPPA
¼
bis(
p
-nitorphenyl) phosphoryl azide.
3.3.4 Aziridination
Chiral cyclic and acyclic allylsulfoxonium ylides are generated from sulfoxonium-substi-
tuted
-amino acids (method A) and 1-alkenylsulfoxonium salts (method
B) upon treatment with DBU (1) [51] (Scheme 3.31). Their application to the asymmetric
aziridination of N-tert-butylsulfonyl imine ester, generated either in situ (method A) or
externally added (method B), affords the corresponding alkenylaziridinecarboxylate with
medium to high diastereoselectivity and enantioselectivity.
g
,
d
-unsaturated
a
3.3.5 Baylis-Hillman Reaction
Amidines catalyse the Baylis-Hillman reaction [52]. A novel one-pot synthesis-kinetic
resolution process involving a DBU (1)-catalysed Baylis-Hillman reaction and a subse-
quent pyridine catalyst/DBU (1)-mediated enantioselective acylation has been developed
[52a] (Scheme 3.32).
3.3.6 Cycloaddition
Enantioselective [3
2] cycloaddition of nitrile imines, which are generated in situ by
dehydrobromination of hydrazonyl bromides with N-crotonyloxazolidinone, has been
developed. On N-arylhydrazonyl bromides, tertiary amines such as triethylamine (Et
3
N),
diisopropylethylamine (
i
Pr
2
NEt) and N-methylmorpholine (NMM) give excellent yields
(90%) and selectivity (94-99% ee). 1,4-Diazabicyclo[2.2.2]octane (DABCO) gives good
selectivity (98% ee) but reduced yield (51%), while both yield and enantioselectivity are
inferior with DBU (1) (60%, 80% ee) and pyridine (37%, 79% ee). However, dehydro-
bromination of N-benzylhydrazonyl bromide did not proceed in the presence of
i
Pr
2
NEt.
Use of DBU (1) enables dipole formation, giving the cycloadduct in 57% yield and 94% ee
[53] (Scheme 3.33).
þ