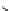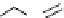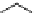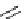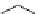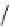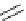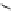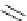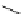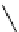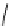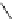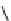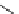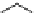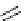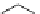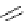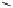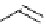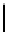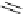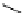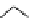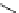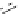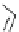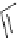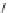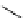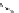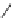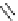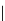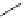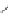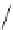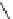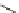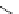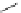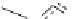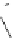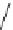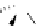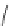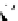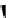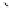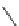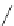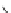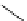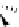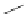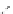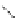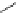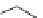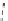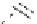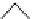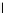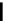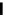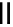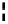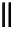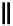Chemistry Reference
In-Depth Information
Me
Me
N
NH
2
N
NH
2
H
H
H
N
N
H
2
N
N
N
N
N
N
N
NH
2
N
NH
2
Me
Me
N
H
2
N
NH
2
NN
NN
N
N
N
N
Me
Me
Me
Me
NN
H
2
N
N
NH
2
N
H
2
N
2
90
89
91
Figure 2.10
Structures of polyguanidines 89-91
amplifies basicity, if it was linked to the highly basic imine nitrogen, which in turnwas a part
of the molecular backbone undergoing aromatization upon protonation (Table 2.9). The
APA of 94 forming a pseudo eight-membered ring (two-centre corona effect, with IMHB on
the imine aminopropyl chain with the neighboring amine) is larger than the pseudo six-
membered IMHB of 93 (single-centre corona effect, the loop starts and ends with the same
atom) by about 2 kcal mol
1
. This difference is caused by ring strain differences and
stronger IMHB of 94 due to better alignment of N
H
þ
...
N atoms.
Aminopropyl substitution of guanidines leads to high intrinsic APAs and basicities
culminating in the APA(MP2) of 92. The reason behind a high PA is a strong cationic
resonance in the central guanidine moiety (the contribution to the PA is in the range
Calculated APAs (kcal mol
-1
) and pK
a
values
Table 2.9
H
R
N
R
N
H
2
N
H
N
H
N
N
N
H
H
H
N
H
N
N
R
H
Me
H
N
N
N
H
N
Me
H
R
N
N
N
N
H
Me
H
N
N
H
H
Me
H
N
R
94H
+
N
R
93H
+
N
H
96
92H
+
: R = Me
95H
+
: R = H
Molecule
PA (MP2)
APA (HF
SC
)
p
K
a
(MeCN)
92
275.5
266.9
29.4
93
254.2
254.5
25.9
94
256.5
256.7
26.8
95
268.4
267.5
28.8
96
275.1
MP2(fc)/6-311
þ
G**//HF/6-31G*
þ
ZPVE(HF/6-31G*);IPCM-B3LYP/6-311
þ
G**//HF/6-31G*.