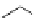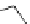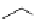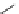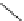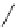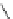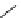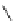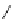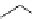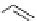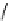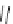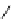Chemistry Reference
In-Depth Information
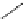
Table 2.4
pK
a
values of amidines with (hetero)ethyl chain
R
1
R
3
N
N
R
2
R
4
NH
R
1
R
2
R
3
R
4
p
K
a
(DMSO)
p
K
a
(H
2
O)
H
H
H
H
11.6
13.6
H
Me
H
H
11.4
13.6
H
H
Me
H
12.0
13.4
H
H
Me
Me
12.0
13.4
Tos
H
H
H
10.8
13.3
Tos
Me
H
H
11.5
13.3
Tos
H
Me
H
11.9
13.3
Tos
H
Me
Me
11.2
12.8
additional lone pair of the 3-aminoethyl (or 3-methoxyethyl) chain enhances the basicity by
stabilization of the conjugate acid (by chelation of the proton and IMHB).
The influence of incorporating an amidine moiety into cyclic structures has also been
studied. Simple bicyclic amidines such as DBN and DBU have larger pK
a
s, compared to
alycyclic amidines (Table 2.5). Five-membered amidine DBN is weaker base compared to
Table 2.5
pK
a
values for cyclic amidines
R
R
R
N
N
R
N
N
N
N
HN
N
N
N
N
N
N
N
N
N
N
N
DBN
63
R
N
N
NH
N
H
N
N
N
N
N
N
65
: R=H
66
: R=Me
67
N
70
68
: R=H
69
: R=Me
DBU
64
Base
p
K
a
(MeCN)
p
K
a
(DMSO)
p
K
a
(H
2
O)
p
K
a
(THF)
p
K
a
(MeCN)
(calcd [49])
70
31.94
31.4
69
30.03
68
29.51
29.8
66
26.95
27.1
65
26.22
67
25.18
64
13-14
DBU
24.33
13.9
16.9 (17.5)
63
24.0
DBN
23.79