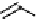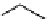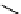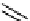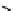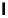Chemistry Reference
In-Depth Information
Me
Me
N
NH
2
NH
martinelline (
49
): R =
Me
NH
O
HN
N
martinellic acid (
50
): R = H
RO
N
N
Me
H
NH
Me
Figure 10.11
Structures of martinelline (49) and martinellic acid (50)
10.4.1 Biguanides
Lowering of blood glucose by the infusion of guanidine [89], biguanides and two linked
guanidine moieties has proved to be useful for the treatment of diabetes mellitus. Three
compounds became available for diabetes therapy, phenformin (51), buformin (52) and
metformin (53) (Figure 10.12). Phenformin (51) was withdrawn due to lactic acidosis [90].
Metformin (53), a less lipophilic biguanide, was recently approved for use in the USA after
20 years of use in Europe [91].
10.4.2 Cimetidine
Black et al. [92] reported the classification and specific blockage of the receptors involved
in mepyramine-insensitive, non-H
1
(H
2
) histamine responses and the discovery of the
selective antagonist brimamide (54), which inhibited histamine-induced gastric acid
secretion and suppressed some other histamine effects not eliminated by H
1
histamine
receptor blockers. Modification of brimamide (54) led to the orally active antagonist
methiamide (55), which proved sufficiently active to allow the exploration of the thera-
peutic potential of this new type of drug. Side effects of kidney damage and agranulocytosis
with methiamide (55) might be attributed to the presence of the thiourea group in the drug
molecule. Owing to the tendency of guanidinohistamine (56) to show weak activity as an
H
2
-receptor antagonist, derivatives with the guanidine moiety were synthesized and,
finally, cimetidine (57), with a cyanoguanidine group with protons in similar acidity as
those of thiourea derivatives such as 54 and 55, was found as an effective histamine H
2
-
receptor antagonist [93]. Famotidine (58) is known as another H
2
-blocker containing
amidine and guanidine parts in the molecule (Figure 10.13).
NH
2
NH
phenformin (
51
) : R
1
= PhCH
2
CH
2
, R
2
= H
buformin (
52
) : R
1
=
n
Bu, R
2
= H
metformin (
53
) : R
1
= R
2
= Me
R
1
NNNH
2
R
2
Figure 10.12
Structures of biguanides