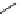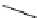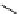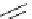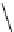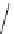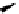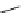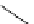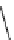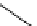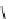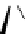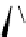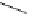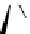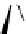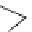Chemistry Reference
In-Depth Information
H
H
Me
Me
Me
Me
N
N
N
N
N
N
n
X
X
X
X
36
(
n
=1-4)
40
: X=H
41
: X=Me
37
: X=H
38
: X=Me
39
: X=Et
Figure 2.4
Structures of polycyclic polyamines 36-41
41 has a minor effect on PAs, while bulkier endo substituents enhance basicity, presumably
due to the buttressing effect, by forcing the NCH
3
groups to be closer to each other, which
increases the lone pair repulsions and, thus, destabilizes the base (Figure 2.4).
Potentiometric titration of azatriquineamine trimer 42 [28] in acetonitrile gave a pK
a
value of 25.1, which is considerably higher than 1.8-diazabicycloundec-7-ene (DBU)
(23.9) and close to phosphazenes. The enhanced basicity of 42 is due to enforced
pyramidalization of the apical nitrogen atom in rigid tricycle, together with a tweezer-
like structure. Calculations indicate that its protonated form has a strong IMHBwith almost
ideal geometrical parameters (N
N distance 2.75 A
H
þ
...
N angle 177
),
...
, and N
contributing to its pronounced superbasicity (Figure 2.5).
Nitrogen bridgehead cyclic polyamines are another structural class of molecules in
which interaction between lone pair electrons is dictated by the carbon framework. Many
polyamines in which two or more nitrogen atoms lie in close proximity show increased
basicity, some of them larger than DMAN (1).
In 1988 Alder reported that the alicyclic diamine 43 was a slightly stronger base than 2
(R
3
R
4
N(CH
3
)
2
,R
5
R
6
11.9) [29]. The 1,6-diazacyclo-
decane framework provides an ideal geometry for a trans-annular hydrogen bond, but there
is not much strain relief when 43 is protonated, since the nitrogen lone pairs in the free base
are accommodated on opposite sides of a relatively strain-free conformation. Following this
work, by use of DFT calculations, Alder designed new, chiral diamines with high pK
a
values, in which strain relief on monoprotonation is the main cause of the extreme
thermodynamic basicity [30]. A more tightly constrained structure than 43 is that of
44: the calculated PA of 44 is much higher than that of 43, due to strain relief on protonation;
¼
¼
¼
¼
OCH
3
) in DMSO (pK
a
¼
N
N
H
H
H
N
H
H
42
Figure 2.5
Structures of azatriquineamine trimer 42