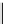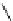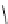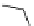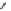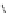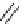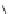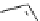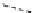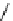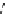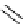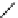Chemistry Reference
In-Depth Information
Ar
S
CO
2
R
RO
2
C
Ar
N
H
RO
2
C
N
H
H
N
Ar
Me
O
Ar
O
N
Me
O
S
H
H
H
N
N
S
Me
Ar
Ar
Scheme 9.4
Supposed mechanism for the thiourea catalysed Claisen rearrangement
Similar thiourea catalysed Claisen rearrangement was theoretically considered by
Kirsten et al. [21]. It was suggested that a transition state is significantly stabilized through
double hydrogen bonding, whereas the overall effect on the barrier is small due to
endergonic conformational changes and complexation (Scheme 9.4).
Urea-type catalyst has to be not only a good hydrogen donor (H-donor) but also a poor
hydrogen acceptor (H-acceptor), in order to avoid self-association which disturbs the
association of urea and a substance (Figure 9.3). Due to its ability toweakly accept hydrogen,
thiourea limits self-association compared to urea. Furthermore, substitution of electron-
withdrawinggroupsat the3and5positionsof thearyl residuenotonlycauses increasedacidity,
in other word hydrogen is liberated easily, but also lowers the hydrogen accepting ability
(Figure 9.5). Amidinium and guanidinium ions, the conjugate acids of organosuperbases,
could serve as strong hydrogen donors. However, they are ineffective catalysts due to their too
strong ability to form hydrogen bonding, in which a catalyst is trapped by product and/or
substrate and cannot be recycled, so-called
. Thus, thiourea has an
advantage in its excellent balance in affinity to product and/or substrate.
Schreiner et al. developed thiourea catalyst as a promising hydrogen donor, which has
more benefit in solubility, synthesis and catalytic turn over number compared with urea
catalyst, in the Diels-Alder reaction of N-crotonyloxazolidinone and cyclopentadiene
[22,23] (Table 9.7). N,N
0
-Di[3,5-bis(trifluoromethyl)phenyl]thiourea accelerates the reac-
tion and improves stereoselectivity (run 4) similar to a metal catalyst such as aluminium
chloride (AlCl
3
) (run 2) or titanium chloride (TiCl
3
) (run 3).
Traces of the reaction by NMR and IR spectra together with ab initio calculations reveals
that the hydrogen bond participated bicyclic structure between the acyloxazolidinone and
thiourea mainly controls the reaction course (Figure 9.6).
The thiourea catalysed Diels-Alder reaction of MVK and cyclopentadiene gives useful
information for further tuning the structure of the thiourea catalyst: flexible side chains on
nitrogen atoms of thiourea are ineffective due to the large entropy in complexation with an
hydrogen acceptor [22] (Table 9.8).
The effect of substitution on the aryl group of thiourea has also been examined (Figure
9.7). Substitution at the ortho position results in a lowering of catalytic activity due to steric
hindrance, and substitution at the para position shows activity that is less effective than
substitution at the meta position.
Trisguanidine is found to recognize phosphate and hydrolyze RNA in an enzyme-like
reaction [24] (Scheme 9.5). Although the use of guanidine as a Lewis acid catalyst is limited
in organic reaction, it may be a potential catalyst under aqueous conditions. Guanidine
catalysed reactions are summarized in Chapter 4.
product inhibition