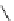Chemistry Reference
In-Depth Information
BnO
BnO
BnO
Ph
O
BnO
BnO
BnO
Ph
O
O
O
O
O
O
O
O
O
BzO
BzO
OAPAC
OH
OMe
OMe
Pd(PPh
3
)
4
(2 mol %)
Proton Sponge™ (
1
)
(1 equiv)
Ph
Ph
EtOH-H
2
O, reflux
O
O
O
O
O
BnO
AcO
O
BnO
AcO
O
O
O
O
BnO
BnO
OAPAC
OH
BnO
OMe
BnO
OMe
Scheme 8.12
Palladium catalysed deprotection of APAC group in the presence of Proton
Sponge (1)
8.6 Concluding Remarks
In this chapter, the synthetic utility of Proton Sponge (1) was reviewed. This superbase,
although not a main player, is indispensable for various mild and selective transformations
in organic synthesis. Despite the unique characteristics of superbases, their exploitation is
still limited. Recently, various types of proton sponges, including chiral ones, have been
developed, and are likely to have a wide range of applications in organic and asymmetric
synthesis.
References
1. Alder, R.W., Bowman, P.S., Steele,W.R.S. andWinterman, D.R. (1968) The remarkable basicity
of 1,8-bis(dimethylamino)naphthalene. Chemical Communications, 723-724.
2. Staab, H.A. and Saupe, T. (1988) 'Proton sponges
and the geometry of hydrogen bonds:
aromatic nitrogen bases with exceptional basicities. Angewandte Chemie - International Edition,
27, 865-879; Alder, R.W. (1989) Strain effects on amine basicities. Chemical Reviews, 89, 1215-
1223.
3. Kim, I.S., Zee, O.P. and Jung, Y.H. (2006) Regioselective and diastereoselective amination of
polybenzyl ethers using chlorosulfonyl isocyanate: total syntheses of 1,4-dideoxy-1,4-imino-
D
-
arabinitol and (
)-lentiginosine. Organic Letters, 8, 4101-4104.
4. Choi, D., Yoo, B., Colson, K.L. et al. (1995) C(10) halogen 10-des(carbamoyloxy)porfir-
omycins - synthesis, chemistry, and biological activity. The Journal of Organic Chemistry, 60,
3391-3396; Regueiro-Ren, A., Borzilleri, R.M., Zheng, X. et al. (2001) Synthesis and
biological activity of novel epothilone aziridines. Organic Letters, 3, 2693-2696.
5. Olofson, R.A., Martz, J.T., Senet J.-P. et al. (1984) A new reagent for the selective, high-yield
N-dealkylation of tertiary-amines - improved synthesis of naltrexone and nalbuphine. The
Journal of Organic Chemistry, 49, 2081-2082.
6. Magnus, P. and Thurston, L.S. (1991) Synthesis of the vinblastine-like antitumor bis-indole
alkaloid navelbine analogue desethyldihydronavelbine. The Journal of Organic Chemistry, 56,
1166-1170; Ratz, A.M. and Weigel, L.O. (1999) S
N
Ar reactions of 2-haloarylsulfoxides with
alkoxides provide a novel synthesis of thiotomoxetine. Tetrahedron Letters, 40, 2239-2242.