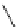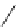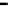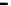Chemistry Reference
In-Depth Information
formation of 209 with LDA, followed by oxidation with MoOPH and successive acetyla-
tion, was cleanly epimerized to the desired C10
-acetate 211 by treatment with DBN in
refluxing toluene in 68% yield with 92% conversion (Scheme 7.49).
Clark et al. reported an efficient synthesis of the A-E fragment of ciguatoxin CTX3C
(216) [62]. Based upon a two-directional and iterative ring closing metathesis (RCM)
strategy, the A-D ring system 212 of CTX3C was obtained. Allylation of the ketone 212
proceeded to give a mixture of 213 and 214 in a ratio of 4 : 1. The desired 214 was generated
as the major product (1 : 4) by an isomerization reaction with Barton
b
s base 5. This
intermediate 214 was successfully converted into the A-E fragment 216 of ciguatoxin
CTX3C via RCM reaction of 215 (Scheme 7.50).
HHH
O
O
O
ABCD
O
O
HHH
OBn
212
1. Me
2
NNH
2
, MgSO
4
,
AcOH-benzene
2.
t
-BuLi, Allylbromide
3. CuCl
2
, THF-H
2
O
44% (3 steps)
213
:
214
= 4 : 1
Barton's Base (
5
), C
6
D
6
, 60 °C
63%
213
:
214
= 1 : 4
HHH
HHH
O
O
O
O
H
H
O
O
+
O
O
O
O
HHH
OBn
HHH
OBn
214
213
PCy
3
Ph
Cl
Cl
Ru
PCy
3
H
H
HHH
HHH
O
O
O
O
E
O
O
(10 mol%)
O
AB
CD
O
H
H
CH
2
Cl
2
, reflux
50%
O
O
O
O
HHH
OBn
HHH
OBn
215
ciguatoxin CTX3C A-E fragment
216
Scheme 7.50
Synthesis of CTX3C A-E fragment 216