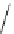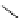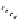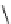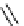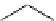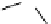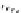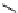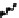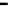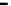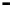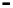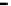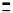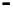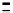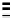Chemistry Reference
In-Depth Information
O
O
Me
Me
Me
H
H
O
i
PrO
2
CCH
2
Br
O
O
O
Me
O
O
Me
H
H
Me
BEMP (
10
)
O
O
Me
O
HO
Me
i
PrO
THF, 95%
145
O
146
1. aq AcOH
2. I
2
, PPh
3
3. DHP, PPTS
56% (3 steps)
THP
I
O
LDA, THF
-78 °C
H
H
O
O
O
THPO
Me
H
O
65%
i
PrO
2
C
O
O
O
Me
Me
H
O
i
PrO
Me
O
148
147
CO
2
H
THP
O
O
H
O
N
H
H
HO
2
C
O
O
H
OH
octosyl acid A (
149
)
Scheme 7.33
Synthesis of octosyl acid A (149)
cysteine thiol group to the orthoquinone methide 181, which was generated from the
a
-hydroxy ketone 180, took place to give the 10-membered lactone 182 by the use of
Barton
s base (5) (Scheme 7.40).
Conjugate addition of hydroperoxide to unsaturated carbonyl compounds generates
epoxide in the presence of superbases. Wood et al. conducted the epoxidation reaction of
183 by treatment with tert-butyl-hydroperoxide, using a catalytic amount of DBU [55]. The
resulting epoxide 184 was converted to (
)-epoxysorbicillinol (185) (Scheme 7.41).
Genki et al. reported the epoxidation reaction of dienone using a combination of tert-
butyl-hydroperoxide and a guanidine base [56]. Applying this methodology, they achieved
a synthesis of (
)-preussomerin L (188) [56]. Reaction of dienone 186 with tert-butyl
hydroperoxide and TBD in toluene gave bis-
-epoxyketone 187 in 91%yield. The ketone
187 was efficiently led to the natural product 188 (Scheme 7.42).
a
,
b