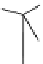
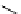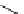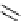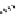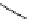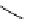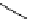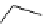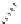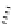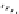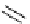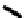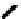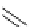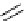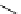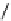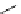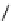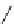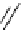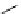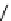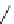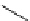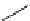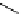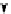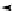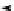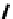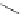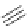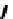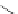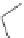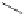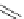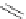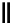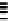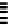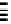Chemistry Reference
In-Depth Information
Me
Me
Me
Me
O
1. NH
2
NH
2
I
2. I
2
,
TMG (
3
)
Me
Me
PivO
PivO
THF, rt
131
132
70%
2.texylborane
3. H
2
O
2
, NaOH
1. Pd(CH
3
CN)
2
, PPh
3
Allyltin, LiCl
Me
Me
Me
Me
OMOM
HO
OH
Me
Me
Me
PivO
OTBDPS
70% (3 steps)
O
KHMDS
44%
OAc
Me
AcO
O
HO
Me
OTBDPS
MOMO
O
H
O
Me
O
Me
Me
H
Me
O
O
Me
H
Me
dumsin (
133
)
Me
Me
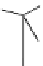
Scheme 7.29
Synthetic studies on dumsin (133)
followed by DBU treatment promoted oxy-Michael reaction to give chromanone 178,
which was further transformed into Sch 57 050 (179) (Scheme 7.39).
Conjugate addition reaction of a thiol group to unsaturated ketonewas efficiently applied
to a synthesis of ecteinascidin 743 (99) by Corey et al. [54]. Conjugate addition of the
O
O
O
O
DBU (
1
)
HBr
3
-Py
O
Br
O
O
O
Br
93%
DMF, 70 °C
73%
CH
2
OH
OPMB
OPMB
OPMB
135
Me
sapinofuranone B (
136
)
134
Scheme 7.30
Synthesis of sapinofuranone B (136)