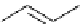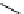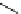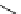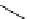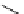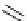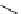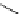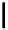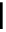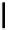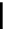Chemistry Reference
In-Depth Information
OMs
DBN (
2
)
O
O
OMe
OMe
O
O
CH
3
CN, 80 °C
64%
Me
O
OMe
Me
O
OH
OMe
OH
NMe
2
NMe
2
AcO
OH
AcO
OAc
ent
-ravidomycin (
115
)
114
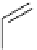
Scheme 7.25
Synthesis of ent-ravidomycin (115)
Similar vinyl iodide formation (131 to 132) was employed by Paquette et al. in synthetic
studies on dumsin (133) [42]. In this synthesis, palladium catalysed Stille coupling was
conducted using the vinyl iodide 132 (Scheme 7.29).
Synthetically useful alkynes can be obtained from 1,2-dibromoalkanes using a variety of
bases. Ohgiya et al. developed the DBU promoted elimination reaction of 1,2-dibromoalk-
anes having an oxygen functional group at C3 [43]. Reaction of 1,2-dibromide 134 having
PMB ether at C3 with DBU in DMF gave alkyne 135 in 73% yield. This intermediate was
employed for the synthesis of sapinofuranone B (136) (Scheme 7.30).
Wender et al. achieved a synthesis of phorbol (140) [44], inwhich the BC-ring system139
was efficiently constructed by transannular [5
2] cycloaddition reaction. Thus, reaction of
acetoxypyranone 137 with DBU in acetonitrile generated the oxidopyrylium intermediate
138 by elimination of the acetoxy group and enolization, and this intermediate smoothly
reacted with alkene to give 139 in 79% yield. This BC-ring system was successfully
converted to phorbol (140) (Scheme 7.31).
Biosynthesis of the polycyclic diterpene intricarene (144) may occur from the natural
product bipinnatin J (141) through transannular [5
þ
2] cycloaddition reaction. Based upon
this proposed biosynthetic route, Tang et al. examined a synthesis of 144 [45]. Synthetic 141
was treated with VO(acac)
2
and tert-butyl hydroperoxide, followed by acetic anhydride to
give acetoxypyranone 142, which was subsequently heated in acetonitrile in the presence of
DBU to give intricarene (144) (Scheme 7.32).
þ
7.5 Ether Synthesis
Ether synthesis by alkylation of alcohol using an inorganic base is sometimes troublesome.
In such cases, soluble organic superbases are often effective. Knapp et al. performed the
alkylation of 145 with isopropyl bromoacetate using the strong soluble base BEMP,
achieving 95% yield [46]. Intramolecular ester enolate alkylation of 147 with LDA took
place to give 148, which was effectively led to the natural product octosyl acid A (149)
(Scheme 7.33).