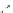Chemistry Reference
In-Depth Information
O
R
63
or
64
(1 equiv)
O
H
OH
R
+
H
O
CH
2
Cl
2
, 4~5 °C
70~94%
MeO
MeO
60a
: R =Me
60b
: R = Et
59
61a
: R =Me (11~26% ee)
61b
: R = Et (30~40% ee)
+
H
H
OH
R
O
MeO
62a
: R =Me (15~33% ee)
62b
: R = Et (40~50% ee)
61
:
62
= 2.5~3.2 : 1
HO
NH
2
CH
3
(CH
2
)
14
B
-
X
4
R''
N
+
H
2
R'
N
OH
63
H
B
-
X
4
N
O
H
O
R
H
H
2
+
N
NH
O
CF
3
X =
CF
3
H
Me
64
Scheme 7.12
Synthesis of (
) norgestrel intermediate
Schuster et al. reported the accelerating effect of the amidinium ion on the Diels-Alder
reaction [15]. Reaction of the diene 59 and diketone 60a or 60b in the presence of lipophilic
amidinium ion 63 (1 equiv.) gave 61 and 62 (2.5
3.2 : 1), with a 100-fold rate increase
compared to the uncatalysed conditions. When the reaction was run in the presence of chiral
amidinium compound 64, 61 and 62 were obtained in 70
94% yield (ca 3 : 1) with
11
50% ee. The Diels-Alder adduct 61b is a key intermediate for synthesis of (
)-
norgestrel (Scheme 7.12). The reaction enhancement effect of amidinium ion can be
explained in terms of the hydrogen bond mediated interaction with diketone.
7.2.4 Wittig Reaction
Organic superbases effectively generate carbonyl-stabilized ylides to promote Wittig
reactions. In the synthesis of (
)-mycalolide A (68) by Panek and Liu, construction of