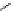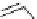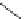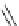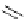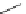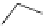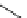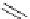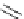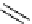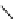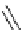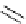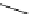Chemistry Reference
In-Depth Information
MOM
N
O
O
N
1. conc. HC, then
i
-Pr
2
NEt, 90%
2. DIBAH
3. Et
3
SiH, TFA
N
MOM
OH
N
N
O
O
O
Me
Me
3
N
Me
CN
65% (2 steps)
O
gelsemine (
28
)
23
27
toluene, reflux
80%
DBU (
1
)
H
2
O
MOM
MOM
O
O
N
N
N
MOM
H
O
3
N
N
N
O
Me
O
OH
Me
Me
CN
CN
NH
24
25
26
Scheme 7.3
Synthesis of gelsemine (28)
The benzofuran skeleton is common in natural products. A direct synthesis from
o-arylmethoxybenzaldehyde by base promoted condensation reaction was reported by
Kraus et al. [6] The reaction of o-arylmethoxybenzaldehyde 29 with 1.1 equiv. of
phosphazene 9 in benzene or pivalonitrile at 90-100
Cgave30 in moderate yield
(Scheme 7.4). Strong ionic bases, such as LDA, LiTMP and KH, were ineffective for this
cyclization reaction.
CHO
R
2
R
2
P
4
-
t
Bu (
9
)
O
O
benzene or
pivalonitrile
80-90 °C
R
1
R
1
R
3
R
3
30
29
47-78%
R
1
= R
2
= R
3
= H
R
1
= R
2
= OMe, R
3
= H
R
1
= R
3
= H, R
2
= OMe
R
1
= R
3
= H, R
2
= OMOM
R
1
= R
2
= H, R
3
= NO
2
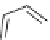
Scheme 7.4
Synthesis of benzofuran skeleton 30