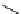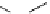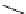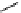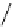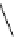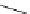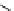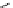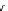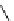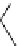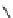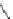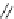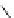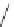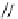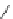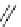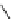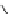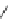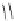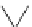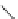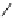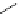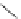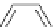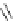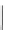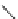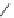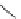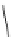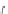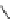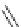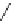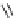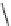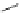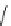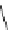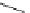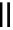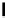Chemistry Reference
In-Depth Information
Me
P
N
Me
NH
H
NN
Me
N
N
N
N
N
Me
Me
N
P
N
N
Me
N
N
N
Me
N
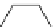
TMG
p
K
a
= 23.7
TBD
p
K
a
= 26.2
DBU
p
K
a
= 23.9
BEMP
p
K
a
= 27.6
PAPT
p
K
a
= 32.9
Figure 6.1
The pK
a
values (in MeCN) of conjugate acids of typical organic superbases
of DBUupon treatment with chloromethylated or
-bromoalkylated polystyrene resins was
highly reliable and gave the desired family of molecules. The synthesis of other reagents of
this type is normally based on this strategy [11,16,17], and some of these polymer-
supported superbase reagents are now available commercially (Figure 6.2).
In this chapter, recent advances in polymer-supported superbase reagents or catalysts in
several organic transformations will be outlined.
o
6.2 Acylation Reactions
Acylation of hydroxyl or amino compounds is important in protective chemistry, and hence
several useful methods have been developed using polymer-supported superbases, relying
primarily on their nature as acid scavengers. In an early stage (1996) of this chemistry,
N
N
N
N
N
N
P
N
N
N
PS-DBU
PS-TBD
PS-BEMP
N
N
3
H
Me
Me
H
Me
Me
P
N
N
P
N
P
N
N
N
N
Me
N
Me
N
N
N
N
PS-NPAPT
PS-N3PAPT
PS-PAPT
= 1-2% cross-linked PS-DVB (Merrifield resin; PS = polystyrene, DVB = divinylbenzene)
Figure 6.2
Representative examples of polymer-supported organic bases