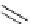Chemistry Reference
In-Depth Information
Table 5.11
Aromatic nucleophilic substitution using malonates
EWG
1
R'
F
t
Bu-P4 (10 mol%)
R'
EWG
1
EWG
2
R
+
R
Et
3
SiH, DMF, 80
o
C
H
EWG
2
R
1
R
2
EWG
1
EWG
2
Run
Time (h)
Yield (%)
1
2-NO
2
Me
CO
2
Et
CO
2
Et
1
99
2
2-NO
2
H
CO
2
Et
CO
2
Et
22
56
n
hexyl
3
2-NO
2
CO
2
Et
CO
2
Et
24
76
4
2-NO
2
allyl
CO
2
Et
CO
2
Et
2
95
5
2-NO
2
allyl
CO
2
Et
CN
3
89
6
2-NO
2
allyl
CN
CN
3
89
7
4-NO
2
Me
CO
2
Et
CO
2
Et
2
97
8
2-CN
Me
CO
2
Et
CO
2
Et
24
46
9
4-CN
Me
CO
2
Et
CO
2
Et
24
66
5.4 Proazaphosphatrane Base (Verkade
s Base)
5.4.1
Properties of Proazaphosphatrane
Proazaphosphatranes are bicyclic, nonionic bases in which the phosphorus atom functions
as the site of electron pair donation. In contrast to phosphazene bases, which are protonated
on a nitrogen atom, proazaphosphatranes are protonated on the bridgehead phosphorus
atomwith a transannulation to form the corresponding azaphosphatranes [60] (Figure 5.7).
The basicity of Verkade
s superbase in acetonitrile solution is shown in that the corre-
sponding pKa value is 29.0. Hence, its basicity is comparable or higher than that of some
other P1 phosphazenes, but it is lower than the basicity of P2 phosphazenes. Structural
characteristics of Verkade
s superbase and its conjugate acid, as well as the origin of its
basicity, have also been examined [61].
5.4.2
Synthesis Using Proazaphosphatrane
5.4.2.1 Activation of Allylsilane
Preparation of homoallylic alcohols was achieved by reacting aromatic aldehydes with
allyltrimethylsilane in the presence of 20mol%
i
Pr-proazaphosphatarene base. Lower
R
H
+
R
R
P
R
N
N
N
H
+
R
R
P
N
N
N
N
N
R = Me,
i
Pr,
i
Bu, Piv
Figure 5.7
Protonation of proazaphosphatranes