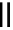Chemistry Reference
In-Depth Information
Table 5.7
Condensation of silylacetate with benzophenone
O
TMSCH
2
CO
2
Et
O
base (10 mol%)
OEt
Ph
Ph
THF, -78
o
C, time
Ph
Ph
Run
Base
Time (h)
Yield (%)
t
Bu-P4 base
1
6
94
t
Bu-P2 base
2
24
0
3
BEMP
24
0
4
DBU
24
0
5
TBAF
6
0
were obtained in good yields (runs 4-6). The double bonds of the products from
arylaldehydes were E geometry. When acetophenone was used as a carbonyl compound,
the reaction with trimethylsilylacetonitrile proceeded smoothly to give the alkene in 63%
yield as a mixture of E and Z isomers (E : Z
89 : 11) (run 7). The reaction of chalcone with
trimethylsilylacetate gave the diene in 81% yield (E : Z
¼
100 : 0) (run 8). Similarly, the
condensation of chalcone with trimethylsilylacetonitrile also gave the corresponding diene
in 80%yield (E : Z
¼
50 : 50) (run 9). As an example of aliphatic aldehyde, hexanal was used
as a substrate and the desired unsaturated ester was obtained in 35% yield [58] (run 10).
Compared to ketones or aldehydes, the reactivities of formamides to nucleophiles were
considered to be lower and the investigation was started with the reaction at room
temperature (Table 5.9). When N-methylformanilide was reacted with ethyl trimethylsi-
lylacetate in THF at room temperature, the condensation proceeded smoothly and the
enaminoester was obtained in 90% yield (run 1). Similarly, trimethylsilylacetonitrile was
¼
Table 5.8
Peterson reaction in the presence of P4
TMSCH
2
EWG
EWG
O
t
Bu-P4 base (10 mol%)
R
1
R
2
THF, -78
o
C, time
R
1
R
2
R
1
R
2
Run
EWG
Time (h)
Yield (%)
1
Ph
Ph
CONEt
2
12
87
2
Ph
Ph
CN
6
78
3
Ph
H
CO
2
Et
6
89
4
4-MeC
6
H
4
H
CO
2
Et
6
91
5
4-MeOC
6
H
4
H
CO
2
Et
6
69
6
2-furyl
H
CO
2
Et
6
85
7
Ph
Me
CN
13
63
8
Ph
CH
¼
CHPh(E)
CO
2
Et
6
81
9
Ph
CH
¼
CHPh(E)
CN
13
80
10
n-Pentyl
H
CO
2
Et
6
35