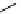Chemistry Reference
In-Depth Information
Table 5.2
P4 catalysed nucleophilic aromatic substitution
F
Nu
Nu
R
catalyst, solvent, rt, time
NO
2
NO
2
Time
(h)
Yield
(%)
Run
Nu
R
Catalyst (mol%)
Solvent
t
Bu-P4 base (10)
1
PhO
H
DMF
1
1.6
t
Bu-P4 base (10)
2
PhO
TMS
DMF
6
quant
t
Bu-P4 base (10)
3
PhO
TBDMS
DMF
1
96
t
Bu-P4 base (10)
4
PhO
TBDMS
DMSO
1
96
2-
t
Bu-C
6
H
4
O
t
Bu-P4 base (10)
5
TBDMS
DMSO
1
99
t
Bu-P4 base (10)
6
2-Br-C
6
H
4
O
TBDMS
DMSO
6
95
t
Bu-P4 base (10)
7
2-I-C
6
H
4
O
TBDMS
DMSO
8
87
t
Bu-P4 base (10)
8
4-MeOC
6
H
4
O
TBDMS
DMSO
1
98
9
PhO
TBDMS
TBAF (10)
DMF
1
trace
t
Bu-P4 base (10)
10
n-HexO
TBDMS
DMSO
24
72
92% yield (run 1). Other bases, such as BEMP or DBU, were found almost inactive as a
promoter (runs 2, 3). p-Fluorobenzonitrile reacted at 100
C to give the desired product in
92%yield (run 4). p-Fluorobenzotrifluoride also reacted at 100
C to give the desired product
in 93% yield (run 5). When the reaction of o-fluorobromobenzene was carried out at 100
C
in DMF, the substitution occurred only at the fluorine substituted position to give the bromo
biaryl ether exclusively in 85% yield (run 6). The reaction of o-iodofluorobenzene was also
examined and the iodophenyl aryl ether was obtained in 43% yield (run 7). The reverse and
complimentary regioselectivity to transition metal catalysed reactions is attractive for
selective functionalization of aromatic compounds [54].
Table 5.3
Synthesis of diaryl ether
F
O
p
-MeOC
6
H
4
OTBDMS
catalyst (10 mol%)
solvent, temp, time
Y
X
Y
X
OMe
Temp (
C)
Run
X
Y
Catalyst
Solvent
Time (h)
Yield (%)
t
Bu-P4 base DMSO
1
H
COOEt
80
2
92
2
H
COOEt
BEMP
DMSO
80
2
1
3
H
COOEt DBU
DMSO
80
2
0
4 HN
t
Bu-P4 base DMSO
100
4
92
t
Bu-P4 base DMSO
5 H F
3
100
10
93
Br H
t
Bu-P4 base DMF
6
100
48
85
I H
t
Bu-P4 base DMF
7
100
48
43