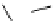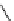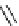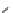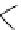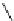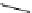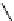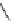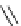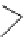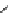Chemistry Reference
In-Depth Information
BnO
BnO
CBz
R
O
CBz
H
O
BnO
BnO
t
Bu-P4
N
N
BnO
BnO
O
O
R-Br
H
H
O
O
d.e. >98%
Scheme 5.24
Diastereoselective alkylation of bicyclic oxazinone
responsible for the depressed yields. The stereochemistry of the products has been
unequivocally ascertained by nuclear Overhauser effect (nOe) measurements and ab initio
calculations [42] (Scheme 5.24).
A synthesis of all four stereoisomers [(1S,2S)-, (1R,2R)-, (1S,2R)- and (1R,2S)-] of
1-amino-2-(hydroxymethyl)cyclobutanecarboxylic acid was presented. The synthesis is
based on the chiral glycine equivalent, employed in both enantiomeric forms. The key step
involves the cyclization of the silyl-protected iodohydrins to the corresponding spiro
derivatives with the aid of the phosphazene base
t
Bu-P4. The final compounds were found
to display moderate potency as ligands for the glycine binding of the N-methyl-D-aspartate
(NMDA) receptor [43] (Scheme 5.25).
Three-membered cyclic sulfones (episulfones) undergo substitution on treatment with
base-electrophile mixtures, such as
t
Bu-P4 phosphazene base-benzyl bromide, to give the
corresponding alkenes following loss of sulfur dioxide (SO
2
). The formation of the
trisubstituted episulfone was observable but the compound proved unstable to the workup
procedure and rapidly decomposed to the alkene as a mixture of stereoisomers [44]
(Scheme 5.26).
Four cyclotetrapeptides containing one or two chiral amino acids have been C-alkylated
or C-hydroxyalkylated through phosphazenium enolates. The reactions are completely
diastereoselectivewith respect to the newly formed backbone stereogenic centres. With the
t
Bu-P4 base, all groups are first benzylated and C-benzylation then takes place at a
sarcosine, rather than an N-benzylglycine residue. In contrast to open chain N-benzyl
peptides, the N-benzylated cyclotetrapeptides could not be debenzylated under dissolving-
metal conditions (Na/NH
3
). Conformational analysis shows that the prevailing species have
cis/trans/cis/trans peptide bonds [45] (Scheme 5.27).
Cyclosporin A can be regioselectively alkylated at the NH of Val-5 with reactive
bromides in the presence of phosphazene base
t
Bu-P4 to yield alkylated products. These
are devoid of immunosuppressive activity in vitro but they have binding affinity for
cyclophilin A and represent a new class of cyclosporin antagonists.
1
H-NMR studies have
shown that the compounds exist in a single, all trans conformation [45c].
5.2.3.2 Generation
a
-Sulfinyl Carbanion
t
Bu-P4 on the yield and diastereoselectivity of additions of thus formed
The effect of
'naked'
-sulfinyl carbanions to butyraldehyde has been studied. Condensation of ethyl
benzylsulfone with butyraldehyde gave a mixture of two expected syn and anti diaster-
eomers. It appeared that the anti isomer was favoured and the diastereoselectivity increased
a