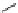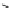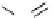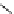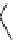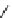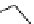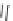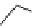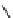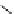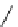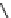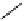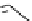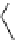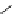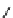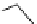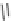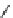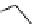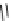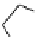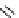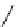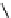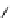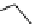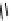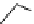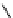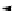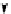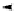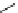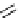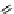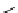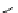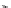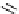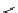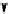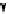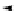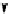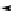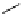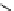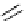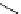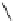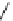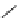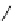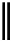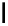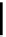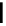Chemistry Reference
In-Depth Information
conjugation of
amidine in A
N
+
HN
NH
N
A
B
A
B
N
+
N
N
N
N
N
N
N
N
N
A
B
C
C
H
+
N
N
conjugation of
vinylogous
guandine in A-C
N
N
C
NH
N
NH
N
A
B
A
B
pentacyclic amidine
N
N
N
N
17.46 (in MeCN)
N
+
N
+
N
N
C
C
Me
Me
N
+
H
NMe
2
NH
NMe
2
Me
H
+
Me
2
N
N
NMe
2
Me
2
N
+
N
NMe
2
N
NMe
2
Me
2
N
N
NMe
2
Me
biguanide
Me
N
+
Me
2
NH
NH
NMe
2
N
+
Me
2
17.1 (H
2
O)
Me
2
N
N
NMe
2
Me
2
N
N
Figure 1.3
Amidine and guanidine derivatives with a vinylogous conjugation system
The examples of simple P1 and P4 bases are shown in Figure 1.5. Their basicity is basically
reflected by the number of the triaminoiminophosphorane units and, thus, P4 bases, the
strongest phosphazene bases, show basicity comparable to organolithium compounds.
Schwesinger et al. [12] reported that the strong basicity of phosphazene bases could be
caused by the efficient distribution of positive charge through conjugation system in the
molecules. However, crystallographic analysis indicates a tetrahedral-like structure around
the phosphorus atom in solid state. Phosphazene bases are easily soluble in common organic
solvents and stable to not only hydrolysis but also attack by electrophiles owing to their
steric bulk [13].
+
H
N
N
+
Me
H
N
NMe
2
N
N
Me
2
N
N
NMe
2
N
N
Figure 1.4
Stabilization effect through hydrogen bonding