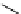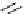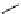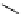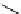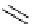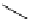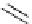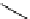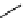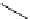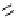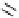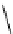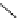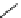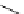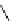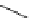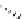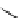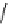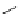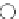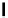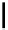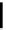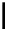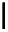Chemistry Reference
In-Depth Information
NR
R
Me
2
N
NMe
2
(Me
2
N)
3
P
NN
P(NMe
2
)
3
N
P
N
R
N
Me
2
N
NMe
2
N
BF
4
N
P
2
Me
2
N
NMe
2
N
R
superbasic bisphosphazene
'proton sponge'
guanidinophosphazene
chiral phosphazene
N=P(NMe
2
)
3
(Me
2
N)
3
P=N
N=P(NMe
2
)
3
N
N
N
N
N
(Me
2
N)
3
P=N
N
N=P(NMe
2
)
3
N=P(NMe
2
)
3
azacalix[3](2,6)pyridine
Figure 5.3
Newly designed phosphazenes
good accordance with the experimental results. They show that the high basicity of HMPN
is a consequence of the high energy content of the base in its initial neutral state and the
intramolecular hydrogen bonding in the resulting conjugate acid with contributions to
proton affinity of 14.1 and 9.5 kcal/mol, respectively [15a]. It is shown by DFT calculation
that HMPN and trisguanidylphosphazene are very powerful neutral organic superbases, as
evidenced by the calculated proton affinities in the gas phase and the corresponding
calculated pKa values in acetonitrile (given within parentheses): 305.4 kcal/mol (44.8) and
287.8 kcal/mol (37.8), respectively [15b].
A chiral example of phosphazene bases was synthesized by treatment of (S)-2-(dialky-
laminomethyl)pyrrolidine derived from 5-oxo-(S)-proline, with phosphorus pentachloride
and subsequent addition of gaseous ammonia. The phosphazenes were isolated as HBF
4
salts in high yields and fully characterized by
1
H,
13
C and
31
P NMR spectroscopy, various
1D and 2DNMR experiments and mass spectrometry (EI). The molecular structure and the
absolute configuration of the HBF
4
salts were determined by X-ray analysis [16].
5.2 Deprotonative Transformations Using Stoichiometric Phosphazenes
In this section, various transformations promoted by the use of stoichiometric phospha-
zenes are discussed, classified by the types of the phosphazenes P1-P4.