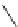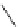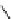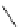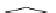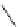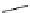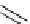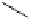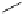Chemistry Reference
In-Depth Information
Table 4.19
Azidation under various conditions
OBn
OBn
OBn
reagents
conditions
MeO
2
C
OTf
MeO
2
C
MeO
2
C
N
3
+
Bn = CH
2
Ph
olefin
azide
Yield (%)
Run
Reagent/solvent
Azide
Olefin
1
NaN
3
/DMF
56
14
2
NaN
3
/DMF-MeCN
33
7
3
Zn(N
3
)
2
(pyridine)
2
/DMF
no reaction
Bn
4
N
þ
N
3
/PhH
4
0
60
5
TMSN
3
/MeCN
complex mixture
6
TMGA (
28
)/DCM
62
7.5
AcO
AcO
TMGA (
28
)
X = Br, Y = OAc: 97% in MeCN; 100% in MeNO
2
X = Cl, Y = NHAc: 95% in MeCN; 95% in MeNO
2
AcO
AcO
H
N
3
AcO
AcO
rt, 1-2.5 h
Y
X
Y
H
AcO
AcO
OAc
OAc
AcO
TMGA (
28
)
AcO
O
O
AcO
H
AcO
N
3
AcO
AcO
AcO
X
AcO
X
AcO
Br
rt, 2 h
AcO
H
99% in MeCN or MeNO
2
Scheme 4.43
TMG (28) mediated azidation of glycosyl halides
Table 4.20
TMGA (28) addition to acetylenic esters
R
1
TMGA (
28
)
R
1
CO
2
R
2
CO
2
R
2
DCM, rt
N
3
R
1
R
2
Run
Time (h)
E
/
Z
Yield (%)
70
a
/30
1
H
Et
36
54
2
MeCO
2
Me
72
0/100
59
3
Ph
Et
72
100/0
85
4
Me
Me
24
70/30
46
a
(
E
)-Ethyl 3-[1-(4-ethoxycarbonyl)-1,2,3-triazoryl]acrylatewas formed as by-product after the 1,3-dipolar cycloaddition of
(
E
)-vinyl azide wirh ethyl propiolate.