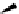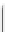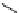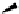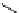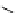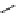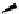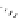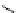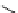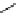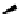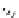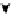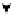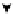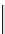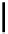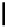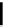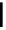Chemistry Reference
In-Depth Information
O
H
C
13
H
27
1 (
S,S
)-
22
TMG / THF
rt, 3 d
2 SiO
2
/ CHCl
3
rt, 3 d
CO
2
t
Bu
on trans
on cis
Bn
N
R
cis: 46% (95% ee)
trans: 41 (97% ee)
H
H
CO
2
t
Bu
CO
2
t
Bu
BnN
BnN
HO
HO
C
13
H
27
C
13
H
27
H
O
O
CH
2
OR
N
RN
O
O
HO
C
13
H
27
CH
2
OH
H
2
N
C
13
H
27
HO
C
13
H
27
Scheme 4.40 Asymmetric synthesis of sphingsine from cis- and trans-aziridines obtained by
guanidinium ylide participating aziridination
Figure 4.7 have been nominated as guanidine-type ionic liquids [103] and used in
hydrogenation [103a,103d,103f], hydroformylation [103b], the aldol reaction [103c,
103e] and the palladium catalysed Heck reaction [103g]. In the last reaction, 2-butyl-
1,1,3,3-tetremethylguanidinium acetate (27) plays multiple roles in the reaction, such as
solvent, a strong base to facilitate
b
-elimination and a ligand to stabilize activated palladium
species.
4.4.3 Tetramethylguanidinium Azide (TMGA)
In 1966 tetramethylguanidinium azide (TMGA) (28) was prepared as an hydroscopic
colourless, but stable, crystal by treatment of TMG (1) with hydrogen azide and was
introduced by Papa [104] as a reactive azidation reagent (Table 4.17).