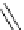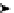Chemistry Reference
In-Depth Information
Table 4.7
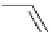
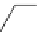
Glycosidation of phenols with 1,2-anhydroglucose derivative
OMe
OMe
conditions
O
O
OMe
OMe
+
HO
X
O
X
O
MeO
MeO
HO
Run
X
Conditions
Yield (%)
a
:
b
1
MeO
ZnCl
2
, DCE
62
55 : 45
2
MeO
ZnCl
2
, TMG (
1
), DCE
73
20 : 80
3
MeO
K
2
CO
3
, THF, 18-crown-6
73
5 : 95
4
NO
2
ZnCl
2
, DCE
60
78 : 22
5
NO
2
ZnCl
2
, TMG (
1
), DCE
83
60 : 40
6
NO
2
K
2
CO
3
, THF, 18-crown-6
60
5 : 95
4.3.2.3 Glycosidation
The zinc chloride (ZnCl
2
) catalysed glycosidation of para-substituted phenols with 1,2-
anhydro-3,4,6-tri-O-methyl-
a
-
D
-glucopyranose gives predominantly the corresponding
a
-
anomer [69]. Addition of TMG (1) enhances the
-selectivity, even to practical completion
under the conditions of potassium carbonate (K
2
CO
3
) and 18-Crown-6, in THF (Table 4.7).
b
4.3.2.4
Intramolecular Substitution (Cyclopropanation)
TMG (1), as well as benzyltrimethylammonium hydroxide (Triton B) in pyridine and
sodium ethoxide in ethanol, was found to work as base catalyst in the cyclopropanation of
steroid skeletons controlled by intramolecular S
N
2 reaction [70]. Thus, 6-oxo-3
a
,5-cyclo-
5
a
-steroids were given in high yields for the reaction of 3
b
-tosyloxy (or -chloro)-6-oxo
derivatives (Table 4.8).
4.3.2.5
Silylation of alcohols
A catalytic amount of TMG (1) effectively works for the silylation of primary and
secondary alcohols with the help of reagents such as tert-butyldimethylchlorosilane
(TBDMCS) in acetonitrile in the co-presence of a stoichiometric amount of tertiary amine
as an acid scavenger [71] (Table 4.9). In the reaction of secondary alcohols, DMF is superior
to acetonitrile as solvent.
Guanidine participating kinetic resolution of 1-indanol with chlorosilane reagents,
TBDMCS or triisopropylchlorosilane (TIPCS) was investigated [72] (Table 4.10). An
(R)-excess silyl ether was afforded as a major enantiomer with moderate ee. The bulkiness
of silylating reagent, as expected, affects the asymmetric induction and 70% ee was
observed in the case of 1-tetrahydrodecanol but yield is low.
4.3.2.6
SNAr Reaction
Barton
s bases are used for the formation of diaryl ether by S
N
Ar reactions [73]. In the
comparison of several bases BTMG (2) was found to be an excellent andmild alternative for
promoting S
N
Ar reactions [73a] (Table 4.11).