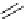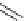Chemistry Reference
In-Depth Information
t
Bu
G =
t
Bu
H
Boc
G
(0.05 mol%)
O
N
O
Boc
NH
N
N
CO
2
Et
+
N
Boc
N
THF
Boc
NH
2
CO
2
Et
100% (97% ee)
t
Bu
t
Bu
Scheme 4.15
Axial guanidine catalysed aza-Michael reaction
4.3.1.8 Michael Reaction
Aza-Michael Reaction
Axially chiral guanidines with an external guanidine unit such as dinaphthoazepineamidine
are effective catalysts for the enantioselective addition of
-oxoesters and a 1,3-diketone to
di-tert-butyl azodicarboxylates to yield
a
-hydrazino-
b
-oxoesters and
a
-hydrazino-
b
-dike-
tones in 54-99% yields and in 15-98% ee [44]. For example, stirring 2-oxocyclopenta-
necarboxylate and di(tert-butyl) azodicarboxylate in THF in the presence of 0.05mol%
catalyst for four hours at
b
60
C provides an adduct in quantitative yield and in 97% ee
(Scheme 4.15). The (R)-catalyst was prepared from (R)-2,2
0
-dimethyl-3,3
0
-binaphthalene-
diol ditriflate, 4-methoxyphenylboronic acid and 3.5-di(tert-butyl)phenylboronic acid in
six steps.
Carba-Michael Reaction
In 1962, Nysted and Burtner [45] reported that the TMG (1) catalysed Michael reaction of
methyl acrylate and 17-nitroandrostane derivative (Scheme 4.16) produced adduct in 84%
yield, whereas the use of Triton B or sodium alkoxide in place of 1 led to low conversion.
This may be the first application of TMG (1) to organic synthesis as an organic base. After
CO
2
Me
NO
2
Me
Me
NO
2
CO
2
Me
Me
Me
TMG (
1
) / benzene
AcO
5 d, rt
AcO
H
H
84%
Scheme 4.16
TMG (1) catalysed Michael reaction of 17-nitroandrostane and acrylate