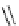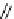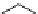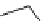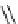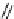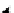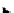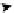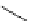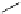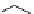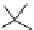Chemistry Reference
In-Depth Information
O
NH
N
Br
O
2
CR
Me
2
N
NMe
2
Br
1
O
-H
+
H
+
+
O
H r
N
+
RCO
2
H
H
N
Me
2
N
NMe
2
O
Scheme 4.13
Proposed catalytic cycle for TMG (1) catalysed bromolactonization
4.3.1.6 Halolactonization
Three organocatalysts, DMF, DMA and TMG (1), were examined for their catalytic roles
for the bromolactonization of
g
,
d
- and
d
,
e
-unsaturated carboxylic acids with N-bromo-
succinimide (NBS). TMG (1) was found to be a superior catalyst for this bromination
(1-10 mol% loading, 100% conversion after 15 min) and to catalyse an intermolecular
bromoacetoxylation of alkenes with acetic acid and NBS. The catalytic cycle is proposed
(Scheme 4.13) [38].
4.3.1.7 Henry (Nitroaldol) Reaction
TMG (1) and TBD derivatives (3), in many cases superior to 1, are proven to be powerful
catalysts for Henry (nitro-aldol) reaction [29,39]. The X-ray structure of the TBD-
phenylnitromethane complex has been reported [39]. Smooth reactions of aldehydes or
ketone with nitroalkanes were observed to afford 2-nitroalkanols under mild conditions
when TMG (1) was used as not only a base but also a solvent [40] (Table 4.4). However,
Table 4.4
TMG (1) catalysed Henry reaction for 2-nitroalkanols
R
3
R
3
CH
2
NO
2
O
CHNO
2
HO
R
1
R
2
TMG (
1
)
R
1
R
2
R
1
R
2
R
3
Time/temp (h/
C)
Run
Yield (%)
1
Ph
H
H
0.5/0
94
2
4-(NO
2
)Ph
H
H
0.25/rt
97
3
4-(MeO)Ph
H
H
1/0
73
4
Pr
H
H
1/rt
67
5
4-(NO
2
)Ph
H
Me
0.25/0
88
6
4-(MeO)Ph
H
Me
1/0
74
7
-(CH
2
)
8
-
Me
48/rt
71