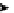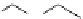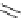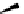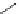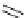Chemistry Reference
In-Depth Information
Table 4.2
Selected Baylis-Hillman reaction of methyl acrylate with various aldehydes
OH
CO
2
Me
CO
2
Me
R
CHO
R
TMG (
1
) / DCM
rt, 6 h
1
Run
R
Mol%
Yield (%)
1
Ph
12.5
49
2
Me
12.5
46
3
Pr
12.5
85
4
Ph(CH
2
)
3
12.5
46
5
(
E
,
E
)-Me(CH
¼
CH)
2
5
85
TMG (1), TBD (3a) and MTBD (3b) act as effective catalysts for the aza-Henry (nitro-
Mannich) reaction of N-diphenylphosphinoyl ketimines and nitromethane. The addition
product is given in good to high yields [32]. Aphophazene
t
Bu-P1
ð
Þ
can be alsoworkable as
a superior base.
4.3.1.3 Baylis-Hillman Reaction
TMG (1) catalyses the Baylis-Hillman reaction [33]. Selected results for the reaction of
aldehydes and methyl acrylate are given in Table 4.2 [33a]. The reactions using aromatic
aldehydes accelerate when either phenol as co-catalyst was added or reaction was
carried out in alcoholic solvent [33b]. The asymmetric version of this reaction remains
unexplored.
4.3.1.4 Concerted-Like Reaction
1,3-Dipolar Addition
A wide range of imines has been reacted with 5-menthyloxyfuranone in acetonitrile
(MeCN) at ambient temperature in a combination of silver acetate with 1,8-diazabicy-
clo[5.4.0]undec-7-ene (DBU) or BTMG (2) to afford 1,3-cycloaddition product in good
yields (71-91%) and high selectivity (de
¼
95%) [34] (Scheme 4.11). BTMG (2)is
superior to DBU.
O
O
OMen
R
2
MenO
O
R
2
O
AgOAc
-
OMe
H
H
OMe
R
1
N
R
1
N
CO
2
Me
BTMG (
2
)
Ag
+
R
1
O
N
R
2
O
i
Pr
MeCN
H
Men =
71-91%
Me
Scheme 4.11
BTMG (2) catalysed 1,3-cycloaddition of imine with 2(5H)-furanone